Page 109 - Photoreactive Organic Thin Films
P. 109
88 ZOUHE1R SEKKAT
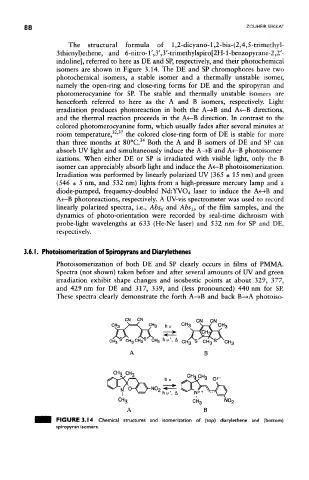
The structural formula of l,2-dicyano-l,2-bis-(2,4,5-trimethyi~
3 thieny 1 )ethene, and 6-nitro-1',3',3 '-trimethylspiro[2H-1 -benzopyrane-2,2'-
indoline], referred to here as DE and SP, respectively, and their photochemical
isomers are shown in Figure 3.14. The DE and SP chromophores have two
photochemical isomers, a stable isomer and a thermally unstable isomer,
namely the open-ring and close-ring forms for DE and the spiropyran and
photomerocyanine for SP. The stable and thermally unstable isomers are
henceforth referred to here as the A and B isomers, respectively. Light
irradiation produces photoreaction in both the A-»B and A4—B directions,
and the thermal reaction proceeds in the A<—B direction. In contrast to the
colored photomerocyanine form, which usually fades after several minutes at
32 37
room temperature, ' the colored close-ring form of DE is stable for more
34
than three months at 80°C. Both the A and B isomers of DE and SP can
absorb UV light and simultaneously induce the A—>B and A<r-B photoisorner-
izations. When either DE or SP is irradiated with visible light, only the B
isomer can appreciably absorb light and induce the A<—B photoisomerization.
Irradiation was performed by linearly polarized UV (365 ± 15 nm) and green
(546 ± 5 nm, and 532 nm) lights from a high-pressure mercury lamp and a
diode-pumped, frequency-doubled Nd:YVO 4 laser to induce the A«->B and
A<—B photoreactions, respectively. A UV-vis spectrometer was used to record
linearly polarized spectra, i.e., Abs// and Abs±, of the film samples, and the
dynamics of photo-orientation were recorded by real-time dichroism with
probe-light wavelengths at 633 (He-Ne laser) and 532 nm for SP and DE,
respectively.
3.6.1. Photoisomerization of Spiropyrans and Diarylethenes
Photoisomerization of both DE and SP clearly occurs in films of PMMA.
Spectra (not shown) taken before and after several amounts of UV and green
irradiation exhibit shape changes and isosbestic points at about 329, 377,
and 429 nm for DE and 317, 339, and (less pronounced) 440 nm for SP.
These spectra clearly demonstrate the forth A-»B and back B—>A photoiso-
CN CN
CH CH 3 h w CH 3 ^ C H 3
Crk
A
FIGURE 3.14 Chemical structures and isomerization of (top) diarylethene and (bottom)
spiropyran isomers.