Page 300 - Physical chemistry understanding our chemical world
P. 300
pH BUFFERS 267
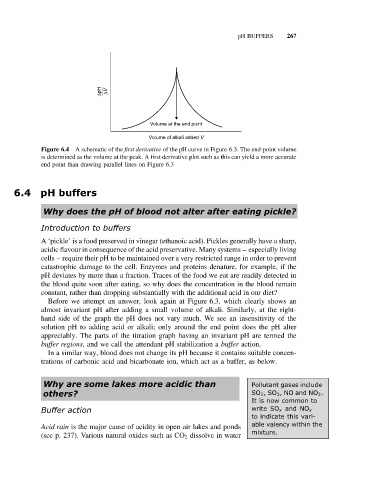
∆pH ∆V
Volume at the end point
Volume of alkali added V
Figure 6.4 A schematic of the first derivative of the pH curve in Figure 6.3. The end-point volume
is determined as the volume at the peak. A first derivative plot such as this can yield a more accurate
end point than drawing parallel lines on Figure 6.3
6.4 pH buffers
Why does the pH of blood not alter after eating pickle?
Introduction to buffers
A ‘pickle’ is a food preserved in vinegar (ethanoic acid). Pickles generally have a sharp,
acidic flavour in consequence of the acid preservative. Many systems – especially living
cells – require their pH to be maintained over a very restricted range in order to prevent
catastrophic damage to the cell. Enzymes and proteins denature, for example, if the
pH deviates by more than a fraction. Traces of the food we eat are readily detected in
the blood quite soon after eating, so why does the concentration in the blood remain
constant, rather than dropping substantially with the additional acid in our diet?
Before we attempt an answer, look again at Figure 6.3, which clearly shows an
almost invariant pH after adding a small volume of alkali. Similarly, at the right-
hand side of the graph the pH does not vary much. We see an insensitivity of the
solution pH to adding acid or alkali; only around the end point does the pH alter
appreciably. The parts of the titration graph having an invariant pH are termed the
buffer regions, and we call the attendant pH stabilization a buffer action.
In a similar way, blood does not change its pH because it contains suitable concen-
trations of carbonic acid and bicarbonate ion, which act as a buffer, as below.
Why are some lakes more acidic than Pollutant gases include
others? SO 2 ,SO 3 ,NO and NO 2 .
It is now common to
Buffer action write SO x and NO x
to indicate this vari-
Acid rain is the major cause of acidity in open-air lakes and ponds able valency within the
mixture.
(see p. 237). Various natural oxides such as CO 2 dissolve in water