Page 301 - Physical chemistry understanding our chemical world
P. 301
268 ACIDS AND BASES
to generate an acid, so the typical pH of normal rainwater is about 5.6; but rainwater
becomes more acidic if pollutants, particularly SO x and NO x , in addition to natural
CO 2 , dissolve in the water. As an example, the average pH of rain in the eastern United
States of America (which produces about one-quarter of the world’s pollution) lies in
the range 3.9–4.5. Over a continental landmass, the partial pressure of SO 2 can be
O
as high as 5 × 10 −9 × p , representing a truly massive amount of pollution.
After rainfall, the pH of the water in some lakes does not change,
Remember: ‘weak’ in whereas others rapidly become too acidic to sustain aquatic life.
this sense indicates the Why? The difference arises from the buffering action of the water.
extent to which a weak Some lakes resist gross change in pH because they contain other
acid dissociates, and chemicals that are able to take up or release protons into solution
does not relate to its following the addition of acid (in the rain). These chemicals in
concentration. the lake help stabilize the water pH, to form a buffer. Look at
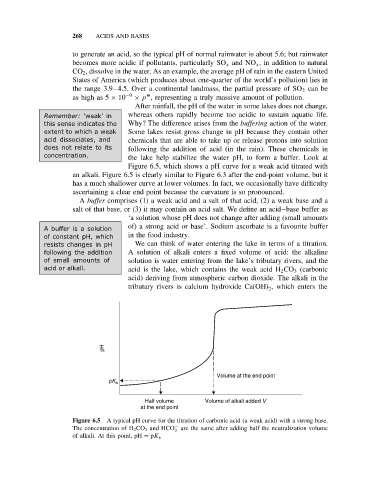
Figure 6.5, which shows a pH curve for a weak acid titrated with
an alkali. Figure 6.5 is clearly similar to Figure 6.3 after the end-point volume, but it
has a much shallower curve at lower volumes. In fact, we occasionally have difficulty
ascertaining a clear end point because the curvature is so pronounced.
A buffer comprises (1) a weak acid and a salt of that acid, (2) a weak base and a
salt of that base, or (3) it may contain an acid salt. We define an acid–base buffer as
‘a solution whose pH does not change after adding (small amounts
A buffer is a solution of) a strong acid or base’. Sodium ascorbate is a favourite buffer
of constant pH, which in the food industry.
resists changes in pH We can think of water entering the lake in terms of a titration.
following the addition A solution of alkali enters a fixed volume of acid: the alkaline
of small amounts of solution is water entering from the lake’s tributary rivers, and the
acid or alkali. acid is the lake, which contains the weak acid H 2 CO 3 (carbonic
acid) deriving from atmospheric carbon dioxide. The alkali in the
tributary rivers is calcium hydroxide Ca(OH) , which enters the
2
pH
Volume at the end point
pK a
Half volume Volume of alkali added V
at the end point
Figure 6.5 A typical pH curve for the titration of carbonic acid (a weak acid) with a strong base.
−
The concentration of H 2 CO 3 and HCO are the same after adding half the neutralization volume
3
of alkali. At this point, pH = pK a