Page 42 - Process Modelling and Simulation With Finite Element Methods
P. 42
FEMLAB and the Basics of Numerical Analysis 29
1.2.2 Root finding: Application to flash distillation
Chemical thermodynamics harbors many common applications of root finding,
since the constraints of chemical equilibrium and mass conservation are
frequently sufficient, along with constitutive models like equations of state, to
provide the same number of constraints as unknowns in the problem. In ths
subsection, we will take flash distillation as an example of simple root finding
for one degree of freedom of the system, which is conveniently taken as the
phase fraction $.
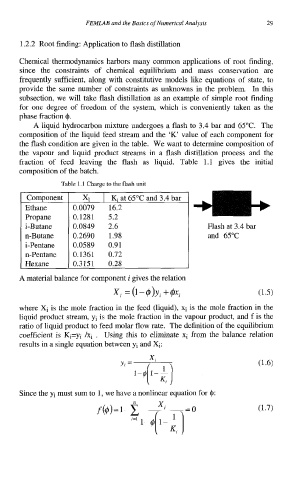
A liquid hydrocarbon mixture undergoes a flash to 3.4 bar and 65°C. The
composition of the liquid feed stream and the 'K' value of each component for
the flash condition are given in the table. We want to determine composition of
the vapour and liquid product streams in a flash distillation process and the
fraction of feed leaving the flash as liquid. Table 1.1 gives the initial
composition of the batch.
Table 1.1 Charge to the flash unit
Propane
&Butane Flash at 3.4 bar
and 65°C
Hexane 0.3151 0.28
A material balance for component i gives the relation
xi = (1 - $)Yi +$Xi
where Xi is the mole fraction in the feed (liquid), xi is the mole fraction in the
liquid product stream, yi is the mole fraction in the vapour product, and f is the
ratio of liquid product to feed molar flow rate. The definition of the equilibrium
coefficient is Ki=yi /xi . Using this to eliminate xi from the balance relation
results in a single equation between yi and Xi:
Since the yi must sum to 1, we have a nonlinear equation for $: