Page 53 - Process Modelling and Simulation With Finite Element Methods
P. 53
40 Process Modelling and Simulation with Finite Element Methods
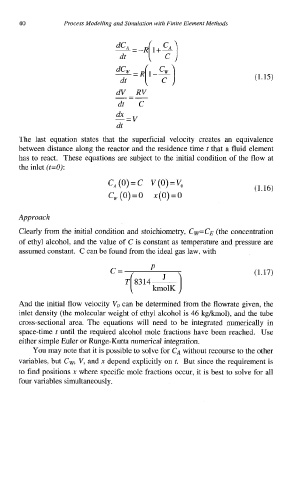
(1.15)
dV - RV
dt C
dx
-=v
dt
The last equation states that the superficial velocity creates an equivalence
between distance along the reactor and the residence time t that a fluid element
has to react. These equations are subject to the initial condition of the flow at
the inlet (t=O):
c*(o)=c V(O)=V,
(1.16)
c, (0) = 0 x(0) = 0
Approach
Clearly from the initial condition and stoichiometry, CW=CE (the concentration
of ethyl alcohol, and the value of C is constant as temperature and pressure are
assumed constant. C can be found from the ideal gas law, with
C= P (1.17)
And the initial flow velocity V, can be determined from the flowrate given, the
inlet density (the molecular weight of ethyl alcohol is 46 kghol), and the tube
cross-sectional area. The equations will need to be integrated numerically in
space-time t until the required alcohol mole fractions have been reached. Use
either simple Euler or Runge-Kutta numerical integration.
You may note that it is possible to solve for CA without recourse to the other
variables, but CW, V, and x depend explicitly on t. But since the requirement is
to find positions x where specific mole fractions occur, it is best to solve for all
four variables simultaneously.