Page 55 - Semiconductor For Micro- and Nanotechnology An Introduction For Engineers
P. 55
The Crystal Lattice System
the two binding atoms a relatively high probability of occupying the
space between the atoms.
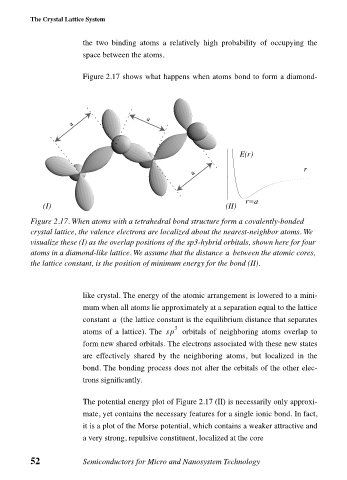
Figure 2.17 shows what happens when atoms bond to form a diamond-
a
a
E(r)
r
a
r=a
(I) (II)
Figure 2.17. When atoms with a tetrahedral bond structure form a covalently-bonded
crystal lattice, the valence electrons are localized about the nearest-neighbor atoms. We
visualize these (I) as the overlap positions of the sp3-hybrid orbitals, shown here for four
atoms in a diamond-like lattice. We assume that the distance between the atomic cores,
a
the lattice constant, is the position of minimum energy for the bond (II).
like crystal. The energy of the atomic arrangement is lowered to a mini-
mum when all atoms lie approximately at a separation equal to the lattice
constant (the lattice constant is the equilibrium distance that separates
a
3
atoms of a lattice). The sp orbitals of neighboring atoms overlap to
form new shared orbitals. The electrons associated with these new states
are effectively shared by the neighboring atoms, but localized in the
bond. The bonding process does not alter the orbitals of the other elec-
trons significantly.
The potential energy plot of Figure 2.17 (II) is necessarily only approxi-
mate, yet contains the necessary features for a single ionic bond. In fact,
it is a plot of the Morse potential, which contains a weaker attractive and
a very strong, repulsive constituent, localized at the core
52 Semiconductors for Micro and Nanosystem Technology