Page 147 - Semiconductor Manufacturing Handbook
P. 147
Geng(SMH)_CH11.qxd 04/04/2005 19:48 Page 11.4
WET ETCHING
11.4 WAFER PROCESSING
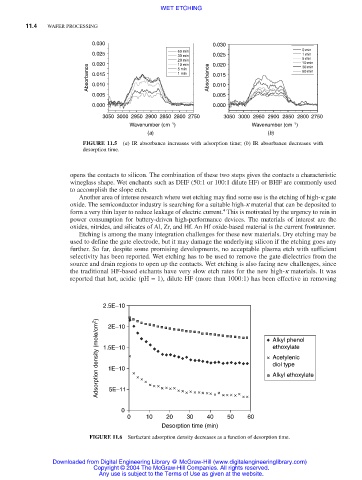
0.030 0.030
0 min
60 min
0.025 30 min 0.025 1 min
20 min 5 min
0.020 10 min 0.020 10 min
Absorbance 0.015 1 min Absorbance 0.015 60 min
30 min
5 min
0.010
0.010
0.005 0.005
0.000 0.000
3050 3000 2950 2900 2850 2800 2750 3050 3000 2950 2900 2850 2800 2750
Wavenumber (cm −1 ) Wavenumber (cm −1 )
(a) (b)
FIGURE 11.5 (a) IR absorbance increases with adsorption time; (b) IR absorbance decreases with
desorption time.
opens the contacts to silicon. The combination of these two steps gives the contacts a characteristic
wineglass shape. Wet enchants such as DHF (50:1 or 100:1 dilute HF) or BHF are commonly used
to accomplish the slope etch.
Another area of intense research where wet etching may find some use is the etching of high-κ gate
oxide. The semiconductor industry is searching for a suitable high-κ material that can be deposited to
form a very thin layer to reduce leakage of electric current. This is motivated by the urgency to rein in
4
power consumption for battery-driven high-performance devices. The materials of interest are the
oxides, nitrides, and silicates of Al, Zr, and Hf. An Hf oxide-based material is the current frontrunner.
Etching is among the many integration challenges for these new materials. Dry etching may be
used to define the gate electrode, but it may damage the underlying silicon if the etching goes any
further. So far, despite some promising developments, no acceptable plasma etch with sufficient
selectivity has been reported. Wet etching has to be used to remove the gate dielectrics from the
source and drain regions to open up the contacts. Wet etching is also facing new challenges, since
the traditional HF-based etchants have very slow etch rates for the new high-κ materials. It was
reported that hot, acidic (pH = 1), dilute HF (more than 1000:1) has been effective in removing
2.5E−10
Adsorption density (mole/cm 2 ) 1.5E−10 Alkyl phenol
2E−10
ethoxylate
Acetylenic
diol type
1E−10
Alkyl ethoxylate
5E−11
0
0 10 20 30 40 50 60
Desorption time (min)
FIGURE 11.6 Surfactant adsorption density decreases as a function of desorption time.
Downloaded from Digital Engineering Library @ McGraw-Hill (www.digitalengineeringlibrary.com)
Copyright © 2004 The McGraw-Hill Companies. All rights reserved.
Any use is subject to the Terms of Use as given at the website.