Page 68 - Separation process engineering
P. 68
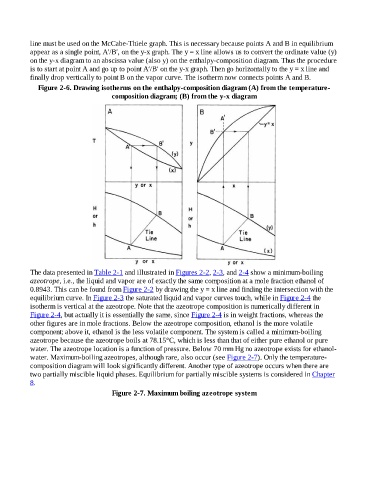
line must be used on the McCabe-Thiele graph. This is necessary because points A and B in equilibrium
appear as a single point, A′/B′, on the y-x graph. The y = x line allows us to convert the ordinate value (y)
on the y-x diagram to an abscissa value (also y) on the enthalpy-composition diagram. Thus the procedure
is to start at point A and go up to point A′/B′ on the y-x graph. Then go horizontally to the y = x line and
finally drop vertically to point B on the vapor curve. The isotherm now connects points A and B.
Figure 2-6. Drawing isotherms on the enthalpy-composition diagram (A) from the temperature-
composition diagram; (B) from the y-x diagram
The data presented in Table 2-1 and illustrated in Figures 2-2, 2-3, and 2-4 show a minimum-boiling
azeotrope, i.e., the liquid and vapor are of exactly the same composition at a mole fraction ethanol of
0.8943. This can be found from Figure 2-2 by drawing the y = x line and finding the intersection with the
equilibrium curve. In Figure 2-3 the saturated liquid and vapor curves touch, while in Figure 2-4 the
isotherm is vertical at the azeotrope. Note that the azeotrope composition is numerically different in
Figure 2-4, but actually it is essentially the same, since Figure 2-4 is in weight fractions, whereas the
other figures are in mole fractions. Below the azeotrope composition, ethanol is the more volatile
component; above it, ethanol is the less volatile component. The system is called a minimum-boiling
azeotrope because the azeotrope boils at 78.15°C, which is less than that of either pure ethanol or pure
water. The azeotrope location is a function of pressure. Below 70 mm Hg no azeotrope exists for ethanol-
water. Maximum-boiling azeotropes, although rare, also occur (see Figure 2-7). Only the temperature-
composition diagram will look significantly different. Another type of azeotrope occurs when there are
two partially miscible liquid phases. Equilibrium for partially miscible systems is considered in Chapter
8.
Figure 2-7. Maximum boiling azeotrope system