Page 212 - Separation process principles 2
P. 212
5.7 Degrees of Freedom and Specifications for Countercurrent Cascades 177
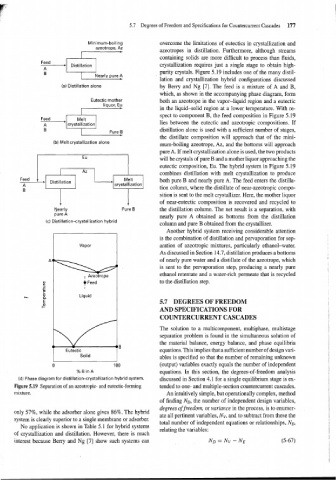
Minimum-boiling overcome the limitations of eutectics in crystallization and
azeotrope, Az azeotropes in distillation. Furthermore, although streams
r----- containing solids are more difficult to process than fluids,
- 1 A Distillation I crystallization requires just a single stage to obtain high-
1 I purity crystals. Figure 5.19 includes one of the many distil-
B
Nearly pure A
lation and crystallization hybrid configurations discussed
(a) Distillation alone by Berry and Ng [7]. The feed is a mixture of A and B,
which, as shown in the accompanying phase diagram, form
Eutectic mother both an azeotrope in the vapor-liquid region and a eutectic
liquor, Eu
in the liquid-solid region at a lower temperature. With re-
spect to component B, the feed composition in Figure 5.19
lies between the eutectic and azeotropic compositions. If
A
B distillation alone is used with a sufficient number of stages,
Pure B
the distillate composition will approach that of the mini-
(b) Melt crystallization alone
mum-boiling azeotrope, Az, and the bottoms will approach
pure A. If melt crystallization alone is used, the two products
will be crystals of pure B and a mother liquor approaching the
eutectic composition, Eu. The hybrid system in Figure 5.19
Distillation 1 crys~~~atioo combines distillation with melt crystallization to produce
1
both pure B and nearly pure A. The feed enters the distilla-
tion column, where the distillate of near-azeotropic compo-
sition is sent to the melt crystallizer. Here, the mother liquor
1 1 of near-eutectic composition is recovered and recycled to
Nearly Pure B the distillation column. The net result is a separation, with
pure A
nearly pure A obtained as bottoms from the distillation
(c) Distillation-crystallization hybrid
column and pure B obtained from the crystallizer.
Another hybrid system receiving considerable attention
is the combination of distillation and pervaporation for sep-
Vapor aration of azeotropic mixtures, particularly ethanol-water.
As discussed in Section 14.7, distillation produces a bottoms
of nearly pure water and a distillate of the azeotrope, which
is sent to the pervaporation step, producing a nearly pure
ethanol retentate and a water-rich permeate that is recycled
, Azeotrope
+ Feed to the distillation step.
I
Liquid
5.7 DEGREES OF FREEDOM
AND SPECIFICATIONS FOR
COUNTERCURRENTCASCADES
The solution to a multicomponent, multiphase, multistage
separation problem is found in the simultaneous solution of
the material balance, energy balance, and phase equilibria
equations. This implies that a sufficient number of design vari-
I Solid I ables is specified so that the number of remaining unknown
0 100 (output) variables exactly equals the number of independent
%BinA equations. In this section, the degrees-of-freedom analysis
(d) Phase diagram for distillation-crystallization hybrid system. discussed in Section 4.1 for a single equilibrium stage is ex-
Figure 5.19 Separation of an azeotropic- and eutectic-forming tended to one- and multiple-section countercurrent cascades.
mixture. An intuitively simple, but operationally complex, method
of finding ND, the number of independent design variables,
degrees offreedom, or variance in the process, is to enumer-
the gives 86% The ate all Nv, and to subtract from these the
57%7
system is clearly superior to a single membrane or adsorber. total number of independent equations or relationships, NE,
No application is shown in Table 5.1 for hybrid systems
relating the variables:
of crystallization and distillation. However, there is much
interest because Berry and Ng [7] show such systems can No = Nv - NE (5-67)