Page 74 - Sustainability in the Process Industry Integration and Optimization
P. 74
P r o c e s s I n t e g r a t i o n f o r I m p r ov i n g E n e r g y E f f i c i e n c y 51
utility. Cooling with water is nonisothermal because the cooling
effect results from sensible heat absorption into the water stream and
thus leads to increasing the temperature.
4.3.1 Setting Energy Targets
Heat Recovery between One Hot and One Cold Stream
The Second Law of thermodynamics states that heat flows from
higher-temperature to lower-temperature regions. As shown in Eq. (4.3),
in a heat exchanger the required heat transfer area is proportional to
the temperature difference between the streams. In HEN design, the
minimum allowed temperature difference (ΔT ) is the lower bound
min
on any temperature difference to be encountered in any heat
exchanger in the network. The value of ΔT is a design parameter
min
determined by exploring the trade-offs between more heat recovery
and the larger heat transfer area requirement. Any given pair of hot
and cold process streams may exchange as much heat as allowed by
their temperatures and the minimum temperature difference.
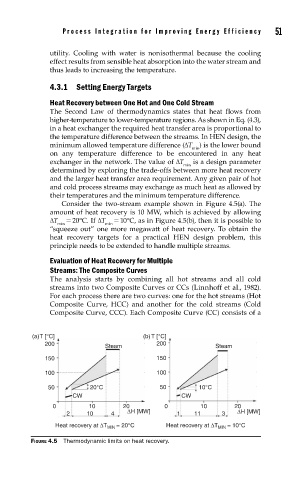
Consider the two-stream example shown in Figure 4.5(a). The
amount of heat recovery is 10 MW, which is achieved by allowing
ΔT = 20°C. If ΔT = 10°C, as in Figure 4.5(b), then it is possible to
min min
“squeeze out” one more megawatt of heat recovery. To obtain the
heat recovery targets for a practical HEN design problem, this
principle needs to be extended to handle multiple streams.
Evaluation of Heat Recovery for Multiple
Streams: The Composite Curves
The analysis starts by combining all hot streams and all cold
streams into two Composite Curves or CCs (Linnhoff et al., 1982).
For each process there are two curves: one for the hot streams (Hot
Composite Curve, HCC) and another for the cold streams (Cold
Composite Curve, CCC). Each Composite Curve (CC) consists of a
(a)T [°C] (b) T [°C]
200 200
Steam Steam
150 150
100 100
50 20°C 50 10°C
CW CW
0 10 20 0 10 20
2 10 4 ΔH [MW] 1 11 3 ΔH [MW]
= 20°C Heat recovery at ΔT = 10°C
Heat recovery at ΔT MIN MIN
FIGURE 4.5 Thermodynamic limits on heat recovery.