Page 283 - Thermodynamics of Biochemical Reactions
P. 283
Thermodynamics of Biocheniical Reactions at Specified pH 283
-23.0327
(c) The standard transformed entropy of reaction at pH 7 and I = 0.25 M is given by
(-23.03+36.07)*1000/298.15
43.7364
(d) The base 10 logarithm of the apparent equilibrium constant is given by
Log[~0.Exp~-dg298pH7is25/(8.31451*.29815)11
6.31308
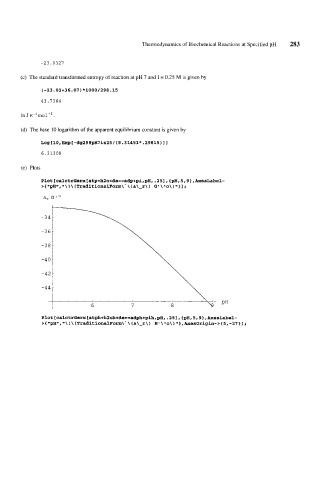
(e) Plots
Plot[calctrGerx[atp+h2o+de==adp+pi,~H,.25],{pH,5,9~,AxesLabel-
\
1
> { tlpH1l, ! \ (TraditionalForm\ . (A\-r\ G' \ ^o\ 'I 1 1 ;
)
I1\
A, G lo
Plot[calctrGerx[atph+h2oh+de==ad~h+p~h,pH,.25l,{pH,5,91,~esLabel-
5, -27 1 I ;
> { llpHtl, ! \ (TraditionalForm\' \ (A\-r\) HI \^o\) ,Axesorigin->{
a*\