Page 285 - Thermodynamics of Biochemical Reactions
P. 285
Thermodynamics of Biochemical Reactions at Specified pH 285
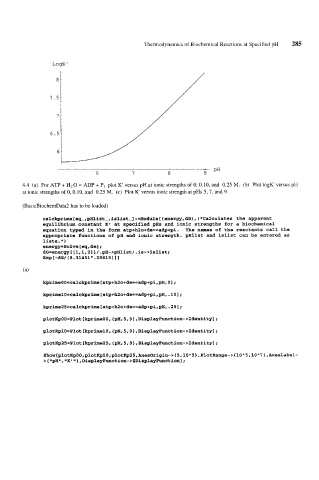
LogK'
'
6 7 8 9 PH
4.4 (a) For ATP + HzO = ADP + Pi plot K versus pH at ionic strengths of 0, 0.10, and 0.25 M. (b) Plot IogK' versus pH
at ionic strengths of 0, 0.10, and 0.25 M. (c) Plot K versus ionic strength at pHs 5, 7, and 9.
(BasicBiochemData2 has to be loaded)
ca~ckprime[e~,pH~ist_,islislist~]:=Module[{energy,dG},(*Calculates apparent
the
equilibrium constant K' at specified pHs and ionic strengths for a biochemical
equation typed in the form atp+hao+de-=adp+pi. The names of the reactants call the
appropriate functions of pH and ionic strength. pHlist and islist can be entered as
lists.*)
energyesolve [eq,de] ;
dG=energy~[l,l,211/.pH->pHlist/.is->islist;
E~p[-dG/(8.31451*.29815)11