Page 710 - Bird R.B. Transport phenomena
P. 710
690 Chapter 22 Interphase Transport in Nonisothermal Mixtures
The mean two phase mass transfer coefficients must be defined carefully, and we con-
sider here only the special case where bulk concentrations in the two adjacent phases do
not change significantly over the total mass-transfer surface S. We may then define K° by
XIU
|
(N ) = J K° , (x - x )dS = K'JXAC ~ (22.4-11)
A0 m x loc Ae Ab
so that, when Eq. 22.3-9 is used,
-dS (22.4-12)
Frequently area mean overall mass transfer coefficients are calculated from area mean
coefficients for the two adjoining phases:
(22.4-13)
х
дг,арргох (I/O + а/т Щ,„)
х
The two mean values in Eqs. 22.4-12 and 13 can be significantly different (see Example
22.4-3).
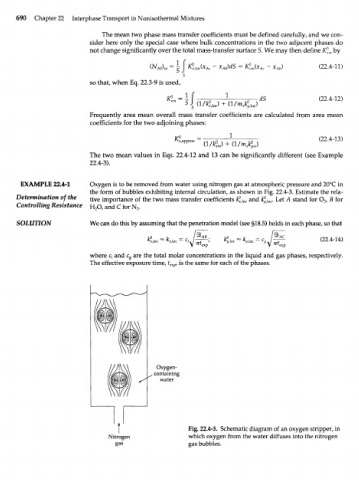
EXAMPLE 22.4-1 Oxygen is to be removed from water using nitrogen gas at atmospheric pressure and 20°C in
the form of bubbles exhibiting internal circulation, as shown in Fig. 22.4-3. Estimate the rela-
Determination of the t i v e importance of the two mass transfer coefficients k° and ^ l o c . Let Л stand for О„ В for
xloc
Controlling Resistance H O, and С for N .
2
2
SOLUTION We can do this by assuming that the penetration model (see §18.5) holds in each phase, so that
t° « k = r, / —• к 0 « к —г / л с СУ) 4-1 А.)
where с, and c are the total molar concentrations in the liquid and gas phases, respectively.
g
The effective exposure time, f , is the same for each of the phases.
exp
Oxygen-
, containing
water
Fig. 22.4-3. Schematic diagram of an oxygen stripper, in
Nitrogen which oxygen from the water diffuses into the nitrogen
gas gas bubbles.