Page 627 - Materials Chemistry, Second Edition
P. 627
CAT3525_C20.qxd 1/27/2005 12:54 PM Page 598
598 Waste Management Practices: Municipal, Hazardous, and Industrial
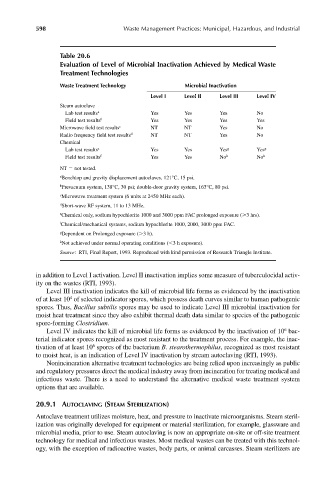
Table 20.6
Evaluation of Level of Microbial Inactivation Achieved by Medical Waste
Treatment Technologies
Waste Treatment Technology Microbial Inactivation
Level I Level II Level III Level IV
Steam autoclave
Lab test results a Yes Yes Yes No
Field test results b Yes Yes Yes Yes
Microwave field test results c NT NT Yes No
Radio frequency field test results d NT NT Yes No
Chemical
Lab test results e Yes Yes Yes g Yes g
Field test results f Yes Yes No h No h
NT not tested.
a Benchtop and gravity displacement autoclaves, 121°C, 15 psi.
b Prevacuum system, 138°C, 30 psi; double-door gravity system, 163°C, 80 psi.
c Microwave treatment system (6 units at 2450 MHz each).
d Short-wave RF system, 11 to 13 MHz.
e Chemical only, sodium hypochlorite 1000 and 3000 ppm FAC prolonged exposure ( 3 hrs).
f Chemical/mechanical systems, sodium hypochlorite 1000, 2000, 3000 ppm FAC.
g Dependent on Prolonged exposure ( 3 h).
h Not achieved under normal operating conditions ( 3 h exposure).
Source: RTI, Final Report, 1993. Reproduced with kind permission of Research Triangle Institute.
in addition to Level I activation. Level II inactivation implies some measure of tuberculocidal activ-
ity on the wastes (RTI, 1993).
Level III inactivation indicates the kill of microbial life forms as evidenced by the inactivation
4
of at least 10 of selected indicator spores, which possess death curves similar to human pathogenic
spores. Thus, Bacillus subtilis spores may be used to indicate Level III microbial inactivation for
moist heat treatment since they also exhibit thermal death data similar to species of the pathogenic
spore-forming Clostridium.
6
Level IV indicates the kill of microbial life forms as evidenced by the inactivation of 10 bac-
terial indicator spores recognized as most resistant to the treatment process. For example, the inac-
6
tivation of at least 10 spores of the bacterium B. stearothermophilus, recognized as most resistant
to moist heat, is an indication of Level IV inactivation by stream autoclaving (RTI, 1993).
Nonincineration alternative treatment technologies are being relied upon increasingly as public
and regulatory pressures direct the medical industry away from incineration for treating medical and
infectious waste. There is a need to understand the alternative medical waste treatment system
options that are available.
20.9.1 AUTOCLAVING (STEAM STERILIZATION)
Autoclave treatment utilizes moisture, heat, and pressure to inactivate microorganisms. Steam steril-
ization was originally developed for equipment or material sterilization, for example, glassware and
microbial media, prior to use. Steam autoclaving is now an appropriate on-site or off-site treatment
technology for medical and infectious wastes. Most medical wastes can be treated with this technol-
ogy, with the exception of radioactive wastes, body parts, or animal carcasses. Steam sterilizers are