Page 288 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 288
P2: KVU/KXT
QC: —/—
P1: KVU/KXT
AT029-Manual-v7.cls
June 22, 2007
AT029-06
AT029-Manual
268 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
oL 10.0 T1: IML 20:46
f r 1.0
Predicted from Eq. 6.190
Data
0.1
0.5 1.0 1.5 2.0 2.5 3.0
Tr
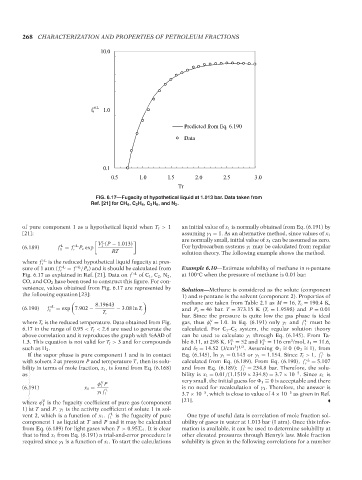
FIG. 6.17—Fugacity of hypothetical liquid at 1.013 bar. Data taken from
Ref. [21] for CH 4 ,C 2 H 4 ,C 2 H 6 , and N 2 .
of pure component 1 as a hypothetical liquid when T r > 1 an initial value of x 1 is normally obtained from Eq. (6.191) by
[21]: assuming γ 1 = 1. As an alternative method, since values of x 1
are normally small, initial value of x 1 can be assumed as zero.
V (P − 1.013)
L
L
(6.189) f = f ◦L P c exp 1 For hydrocarbon systems γ 1 may be calculated from regular
1 r RT solution theory. The following example shows the method.
where f r ◦L is the reduced hypothetical liquid fugacity at pres-
◦L
sure of 1 atm ( f r ◦L = f /P c ) and it should be calculated from Example 6.10—Estimate solubility of methane in n-pentane
Fig. 6.17 as explained in Ref. [21]. Data on f ◦L of C 1 ,C 2 ,N 2 , at 100 C when the pressure of methane is 0.01 bar.
◦
CO, and CO 2 have been used to construct this figure. For con-
venience, values obtained from Fig. 6.17 are represented by Solution—Methane is considered as the solute (component
the following equation [23]: 1) and n-pentane is the solvent (component 2). Properties of
8.19643 methane are taken from Table 2.1 as M = 16, T c = 190.4K,
(6.190) f r ◦L = exp 7.902 − − 3.08 ln T r and P c = 46 bar. T = 373.15 K (T r = 1.9598) and P = 0.01
T r
bar. Since the pressure is quite low the gas phase is ideal
V
where T r is the reduced temperature. Data obtained from Fig. gas, thus φ = 1.0. In Eq. (6.191) only γ 1 and f 1 L must be
1
6.17 in the range of 0.95 < T r < 2.6 are used to generate the calculated. For C 1 –C 5 system, the regular solution theory
above correlation and it reproduces the graph with %AAD of can be used to calculate γ 1 through Eq. (6.145). From Ta-
L
3
L
1.3. This equation is not valid for T r > 3 and for compounds ble 6.11, at 298 K, V = 52 and V = 116 cm /mol, δ 1 = 11.6,
1 2
3 1/2
such as H 2 . and δ 2 = 14.52 (J/cm ) . Assuming 1 = 0( 2 = 1), from
∼
∼
If the vapor phase is pure component 1 and is in contact Eq. (6.145), ln γ 1 = 0.143 or γ 1 = 1.154. Since T r > 1, f 1 L is
with solvent 2 at pressure P and temperature T, then its solu- calculated from Eq. (6.189). From Eq. (6.190), f r ◦L = 5.107
L
bility in terms of mole fraction, x 1 , is found from Eq. (6.168) and from Eq. (6.189): f = 234.8 bar. Therefore, the solu-
1
−5
as bility is x 1 = 0.01/(1.1519 × 234.8) = 3.7 × 10 . Since x 1 is
very small, the initial guess for 1 = 0 is acceptable and there
∼
V
φ P
(6.191) x 1 = 1 is no need for recalculation of γ 1 . Therefore, the answer is
γ 1 f L −5 −5
1 3.7 × 10 , which is close to value of 4 × 10 as given in Ref.
V
where φ is the fugacity coefficient of pure gas (component [21].
1
1) at T and P. γ 1 is the activity coefficient of solute 1 in sol-
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
vent 2, which is a function of x 1 . f 1 L is the fugacity of pure One type of useful data is correlation of mole fraction sol-
component 1 as liquid at T and P and it may be calculated ubility of gases in water at 1.013 bar (1 atm). Once this infor-
from Eq. (6.189) for light gases when T > 0.95T c1 . It is clear mation is available, it can be used to determine solubility at
that to find x 1 from Eq. (6.191) a trial-and-error procedure is other elevated pressures through Henry’s law. Mole fraction
required since γ 1 is a function of x 1 . To start the calculations solubility is given in the following correlations for a number
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT