Page 292 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 292
QC: —/—
P2: KVU/KXT
P1: KVU/KXT
AT029-Manual-v7.cls
June 22, 2007
AT029-Manual
AT029-06
272 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
Pressure, PSIA
30
10 T1: IML 4 50 6 7 8 9100 20:46 300 4 500 6 7 8 91,000 2 3,000 4 6 7 8 9 10,000
2
2
1,000 1,000
9 9
8 8
7 7
6 6
5 5
4 4
3 3
2 2
100 100
9 9
8 8
7 7
6 6
5 5
4 4
3 3
2 2
10 Temperature °F 10
9 9
500
8 400 8
7 300 7
6 250 6
5 200 5
4 4
K = y /x 3 100 3 K = y /x
2 80 2
60 40
1.0 20 1.0
0
9 9
8 –20 8
7 7
6 –40 6
5 5
4 4
3 3
2 2
0.1 0.1
9 9
8 8
7 7
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
6 6
5 5
4 4
3 3
2 2
.01 .01
10 2 30 4 50 6 7 8 9 100 2 300 4 500 6 7 8 9 1,000 2 3,000 4 6 7 8 9 10,000
Pressure, PSIA Ethane
CONV. PRESS. 10,000 PSIA
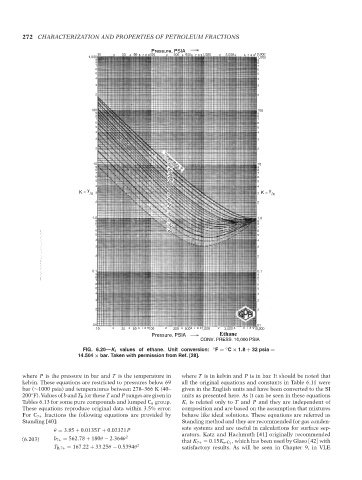
FIG. 6.20—K i values of ethane. Unit conversion: ◦ F = C × 1.8 + 32 psia =
◦
14.504 × bar. Taken with permission from Ref. [28].
where P is the pressure in bar and T is the temperature in where T is in kelvin and P is in bar. It should be noted that
kelvin. These equations are restricted to pressures below 69 all the original equations and constants in Table 6.11 were
bar (∼1000 psia) and temperatures between 278–366 K (40– given in the English units and have been converted to the SI
200 F). Values of band T B for these T and P ranges are given in units as presented here. As it can be seen in these equations
◦
Tables 6.13 for some pure compounds and lumped C 6 group. K i is related only to T and P and they are independent of
These equations reproduce original data within 3.5% error. composition and are based on the assumption that mixtures
For C 7+ fractions the following equations are provided by behave like ideal solutions. These equations are referred as
Standing [40]: Standing method and they are recommended for gas conden-
θ = 3.85 + 0.0135T + 0.02321P sate systems and are useful in calculations for surface sep-
arators. Katz and Hachmuth [41] originally recommended
(6.203) b 7+ = 562.78 + 180θ − 2.364θ 2
that K 7+ = 0.15K n-C 7 , which has been used by Glaso [42] with
T B,7+ = 167.22 + 33.25θ − 0.5394θ 2 satisfactory results. As will be seen in Chapter 9, in VLE
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT