Page 297 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 297
QC: —/—
P2: KVU/KXT
P1: KVU/KXT
AT029-06
AT029-Manual-v7.cls
AT029-Manual
20:46
June 22, 2007
Pressure, PSIA
67 8 9 100
6 7 8 9 10,000
67 8 9 1,000
4 50
10 T1: IML 30 6. THERMODYNAMIC RELATIONS FOR PROPERTY ESTIMATIONS 277
4 500
3,000
300
2
4
100 2 2 100
9 9
8 8
7 7
6 6
5 5
4 4
3 3
2 2
10 10
9 9
8 8
7 7
6 6 --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
5 5
4 4
3 Temperature °F 3
2 500 2
450
400
1.0 1.0
9 300 9
8 280 8
7 7
6 260 6
5 240 5
4 220 4 y
K = y /x 3 200 3 K = /x
180
2 160 2
140
0.1 120 0.1
9 100 9
8 8
7 7
6 80 6
5 5
60
4 4
3 40 3
2 2
20
.01 0 .01
9 9
8 8
7 7
6 –20 6
5 5
4 4
–40
3 3
2 2
.001 .001
10 2 30 4 50 67 8 9 100 2 300 4 500 67 8 9 1,000 2 3,000 4 6 7 8 9 10,000
Pressure, PSIA n - Pentane
CONV. PRESS. 10,000 PSIA
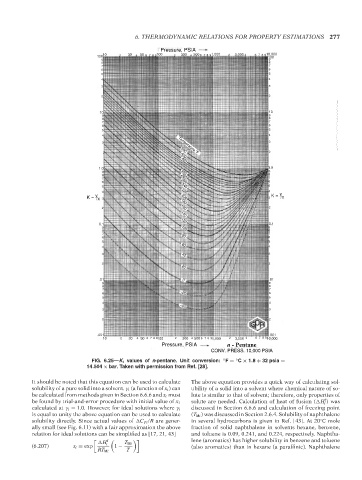
FIG. 6.25—K i values of n-pentane. Unit conversion: F = C × 1.8 + 32 psia =
◦
◦
14.504 × bar. Taken with permission from Ref. [28].
It should be noted that this equation can be used to calculate The above equation provides a quick way of calculating sol-
solubility of a pure solid into a solvent. γ i (a function of x i ) can ubility of a solid into a solvent where chemical nature of so-
be calculated from methods given in Section 6.6.6 and x i must lute is similar to that of solvent; therefore, only properties of
f
be found by trial-and-error procedure with initial value of x 1 solute are needed. Calculation of heat of fusion ( H ) was
i
calculated at γ i = 1.0. However, for ideal solutions where γ i discussed in Section 6.6.6 and calculation of freezing point
is equal to unity the above equation can be used to calculate (T Mi ) was discussed in Section 2.6.4. Solubility of naphthalene
solubility directly. Since actual values of C Pi /R are gener- in several hydrocarbons is given in Ref. [43]. At 20 C mole
◦
ally small (see Fig. 6.11) with a fair approximation the above fraction of solid naphthalene in solvents hexane, benzene,
relation for ideal solutions can be simplified as [17, 21, 43] and toluene is 0.09, 0.241, and 0.224, respectively. Naphtha-
H T Mi
f lene (aromatics) has higher solubility in benzene and toluene
(6.207) x i = exp i 1 − (also aromatics) than in hexane (a paraffinic). Naphthalene
RT Mi T
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT