Page 300 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 300
P2: KVU/KXT
QC: —/—
P1: KVU/KXT
20:46
June 22, 2007
AT029-Manual-v7.cls
AT029-06
AT029-Manual
280 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
6 7 8 9 1,000
10 T1: IML 4 50 67 8 9100 Pressure, PSIA 2 3,000 4 6 7 8 910,000
30
4 500
300
10 2 2 10
9 Plotted from 1947 tabulations of 9
8 G. G. Brown, University of Michigan. 8
7 Extrapolated and drawn by 7
6 The Fluor Corp. Ltd. in 1957. 6
5 5
4 4
3 3
2 2
1.0 Temperature °F 1.0
9 500 9
8 8
7 450 7
6 6
5 5
4 380 4
400
3 360 3
2 340 2
320
300
0.1 280 0.1
9 260 9
8 8
7 7
6 240 6
5 220 5
4 4
K = y /x 3 200 3 K = y /x
2 180 2
160
.01 140 .01
9 9
8 8
7 120 7
6 6
5 5
4 100 4
3 80 3
2 2
60
.001 40 .001 --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
9 9
8 8
7 7
6 20 6
5 5
4 4
3 0 3
2 2
–20
.0001 .0001
10 2 30 4 50 67 8 9 100 2 300 4 500 6 7 8 91,000 2 3,000 4 6 7 8 9 10,000
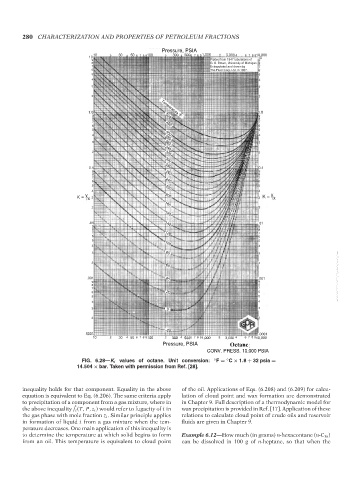
Pressure, PSIA Octane
CONV. PRESS. 10,000 PSIA
FIG. 6.28—K i values of octane. Unit conversion: ◦ F = C × 1.8 + 32 psia =
◦
14.504 × bar. Taken with permission from Ref. [28].
inequality holds for that component. Equality in the above of the oil. Applications of Eqs. (6.208) and (6.209) for calcu-
equation is equivalent to Eq. (6.206). The same criteria apply lation of cloud point and wax formation are demonstrated
to precipitation of a component from a gas mixture, where in in Chapter 9. Full description of a thermodynamic model for
ˆ
the above inequality f i (T, P, z i ) would refer to fugacity of i in wax precipitation is provided in Ref. [17]. Application of these
the gas phase with mole fraction z i . Similar principle applies relations to calculate cloud point of crude oils and reservoir
in formation of liquid i from a gas mixture when the tem- fluids are given in Chapter 9.
perature decreases. One main application of this inequality is
to determine the temperature at which solid begins to form Example 6.12—How much (in grams) n-hexacontane (n-C 36 )
from an oil. This temperature is equivalent to cloud point can be dissolved in 100 g of n-heptane, so that when the
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT