Page 301 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 301
QC: —/—
P2: KVU/KXT
P1: KVU/KXT
AT029-Manual
AT029-Manual-v7.cls
AT029-06
20:46
June 22, 2007
Pressure, PSIA
6 7 8 9 10,000
6 7 8 9 1,000
67 8 9100
10 T1: IML 30 6. THERMODYNAMIC RELATIONS FOR PROPERTY ESTIMATIONS 281 --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
4 50
300
4 500
3,000
4
2
10 2 2 10
9 Plotted from 1947 tabulations of 9
8 G. G. Brown, University of Michigan. 8
7 Extrapolated and drawn by 7
6 The Fluor Corp. Ltd. in 1957. 6
5 5
4 4
3 3
2 2
1.0 1.0
9 9
8 Temperature °F 8
7 500 7
6 6
5 5
4 450 4
3 400 3
2 380 2
360
0.1 340 0.1
320
9 9
8 300 8
7 7
6 280 6
5 5
4 260 4
K = y /x 3 240 3 K = y /x
2 220 2
.01 200 .01
9 180 9
8 8
7 160 7
6 6
5 5
4 140 4
3 120 3
2 100 2
.001 80 .001
9 9
8 8
7 60 7
6 6
5 5
4 40 4
3 3
2 20 2
0 0
.0001 .0001
10 2 30 4 50 67 8 9 100 2 300 4 500 6 7 8 91,000 2 3,000 4 6 7 8 910,000
Pressure, PSIA Nonane
CONV. PRESS. 10,000 PSIA
◦
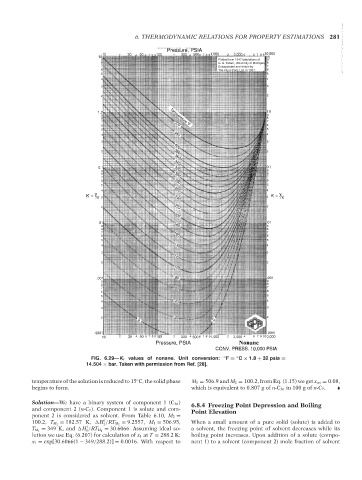
FIG. 6.29—K i values of nonane. Unit conversion: ◦ F = C × 1.8 + 32 psia =
14.504 × bar. Taken with permission from Ref. [28].
temperature of the solution is reduced to 15 C, the solid phase M 1 = 506.9 and M 2 = 100.2, from Eq. (1.15) we get x wi = 0.08,
◦
begins to form. which is equivalent to 0.807 g of n-C 36 in 100 g of n-C 7 .
Solution—We have a binary system of component 1 (C 36 ) 6.8.4 Freezing Point Depression and Boiling
and component 2 (n-C 7 ). Component 1 is solute and com-
Point Elevation
ponent 2 is considered as solvent. From Table 6.10, M 2 =
f
2
100.2, T M 2 = 182.57 K, H /RT M 2 = 9.2557, M 1 = 506.95, When a small amount of a pure solid (solute) is added to
f
1
T M 1 = 349 K, and H /RT M 1 = 30.6066. Assuming ideal so- a solvent, the freezing point of solvent decreases while its
lution we use Eq. (6.207) for calculation of x 1 at T = 288.2K: boiling point increases. Upon addition of a solute (compo-
x 1 = exp[30.6066(1 − 349/288.2)] = 0.0016. With respect to nent 1) to a solvent (component 2) mole fraction of solvent
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT