Page 298 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 298
QC: —/—
P2: KVU/KXT
P1: KVU/KXT
June 22, 2007
20:46
AT029-Manual
AT029-Manual-v7.cls
AT029-06
278 CHARACTERIZATION AND PROPERTIES OF PETROLEUM FRACTIONS
4 500
10 T1: IML 4 50 67 8 9100 Pressure, PSIA 67 8 9 1,000 2 3,000 4 6 7 8 9 10,000
300
30
2
2
10 10
9 9
8 8
7 7
6 6
5 5
4 4
3 3
2 Temperature °F 2
500
1.0 450 1.0
9 400 9
8 8
7 7
6 6
5 300 5
4 280 4
3 260 3
240
2 220 2
200
0.1 180 0.1
9 9
8 160 8
7 7
6 140 6
5 5
4 120 4
K = y /x 3 100 3 K = y /x
2 80 2
60
.01 .01
9 40 9
8 8
7 7
6 6
20
5 5
4 4
0
3 3
2 2
–20
.001 .001
9 –40 9
8 8
7 7
6 6
5 5
4 4
3 3
2 2
.0001 .0001
10 2 30 4 50 67 8 9100 2 300 4 500 67 8 9 1,000 2 3,000 4 67 8 9 10,000
Pressure, PSIA Hexane
CONV. PRESS. 10,000 PSIA
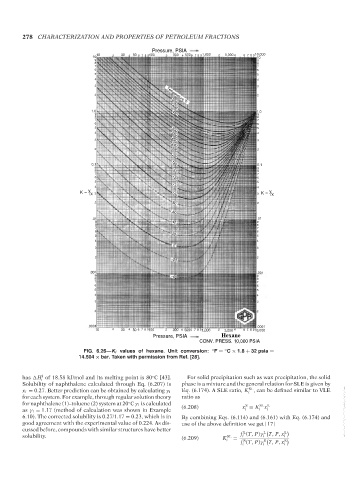
FIG. 6.26—K i values of hexane. Unit conversion: ◦ F = C × 1.8 + 32 psia =
◦
14.504 × bar. Taken with permission from Ref. [28].
f
has H of 18.58 kJ/mol and its melting point is 80 C [43]. For solid precipitation such as wax precipitation, the solid
◦
i
Solubility of naphthalene calculated through Eq. (6.207) is phase is a mixture and the general relation for SLE is given by
SL
Eq. (6.174). A SLE ratio, K , can be defined similar to VLE
x 1 = 0.27. Better prediction can be obtained by calculating γ 1 i
for each system. For example, through regular solution theory ratio as
for naphthalene (1)–toluene (2) system at 20 C γ 1 is calculated S SL L
◦
i
as γ 1 = 1.17 (method of calculation was shown in Example (6.208) x = K i x i --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
6.10). The corrected solubility is 0.27/1.17 = 0.23, which is in By combining Eqs. (6.114) and (6.161) with Eq. (6.174) and
good agreement with the experimental value of 0.224. As dis- use of the above definition we get [17]
cussed before, compounds with similar structures have better L L L
solubility. (6.209) K SL f (T, P)γ i T, P, x i
i
i = S S S
f (T, P)γ T, P, x
i i i
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT