Page 295 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 295
P2: KVU/KXT
QC: —/—
P1: KVU/KXT
AT029-Manual-v7.cls
20:46
AT029-Manual
June 22, 2007
AT029-06
Pressure, PSIA
6 7 8 9 100
4 50
10 T1: IML 30 6. THERMODYNAMIC RELATIONS FOR PROPERTY ESTIMATIONS 275
300
4 500
6 7 8 9 1,000
6 7 8 9 10,000
3,000
4
2
2
2
100 100
9 9
8 8
7 7
6 6
5 5
4 4
3 3
2 2
10 10
9 9
8 8
7 7
6 6
5 Temperature °F 5
4 500 4
3 450 3
400
2 350 2
300
1.0 1.0
9 200 9
8 8
7 180 7
6 160 6
5 140 5
4 120 4
K = y K = y
/x 3 100 3 /x
2 80 2
60
0.1 40 0.1
9 9
8 8
7 20 7
6 6
5 5
0
4 4
3 –20 3
2 –40 2
.01 .01
9 9
8 8
7 7
6 6
5 5
4 4
3 3
2 2
.001 .001
10 2 30 4 50 6 7 8 9 100 2 300 4 500 6 7 8 9 1,000 2 3,000 4 6 7 8 9 10,000
Pressure, PSIA n - Butane
CONV. PRESS. 10,000 PSIA
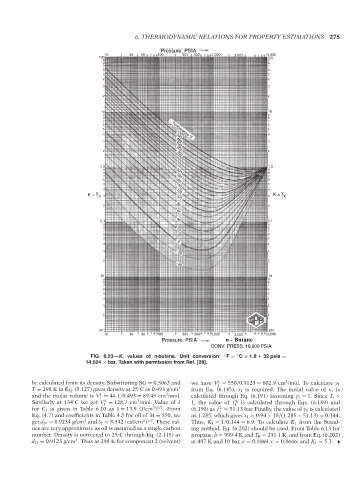
FIG. 6.23—K i values of n-butane. Unit conversion: ◦ F = C × 1.8 + 32 psia =
◦
14.504 × bar. Taken with permission from Ref. [28].
L
3
be calculated from its density. Substituting SG = 0.5063 and we have V = 550/0.9123 = 602.9cm /mol. To calculate γ 1
2
T = 298 K in Eq. (5.127) gives density at 25 C as 0.493 g/cm 3 from Eq. (6.145), x 1 is required. The initial value of x 1 is
◦
3
L
and the molar volume is V = 44.1/0.493 = 89.45 cm /mol. calculated through Eq. (6.191) assuming γ 1 = 1. Since T r >
1
3
L
Similarly at 134 Cweget V = 128.7cm /mol. Value of δ 1, the value of f L is calculated through Eqs. (6.189) and
◦
1 1
L
3 1/2
for C 3 is given in Table 6.10 as δ = 13.9 (J/cm ) . From (6.190) as f = 51.13 bar. Finally, the value of γ 1 is calculated
1
Eq. (4.7) and coefficients in Table 4.5 for oil of M = 550, we as 1.285, which gives x 1 = 0.94 × 10/(1.285 × 51.13) = 0.144. --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
3 1/2
3
get d 20 = 0.9234 g/cm and δ 2 = 8.342 (cal/cm ) . These val- Thus, K 1 = 1/0.144 = 6.9. To calculate K 1 from the Stand-
ues are very approximate as oil is assumed as a single carbon ing method, Eq. (6.202) should be used. From Table 6.13 for
number. Density is corrected to 25 C through Eq. (2.115) as propane, b = 999.4 K and T B = 231.1 K, and from Eq. (6.202)
◦
3
d 25 = 0.9123 g/cm . Thus at 298 K for component 2 (solvent) at 407 K and 10 bar, a = 0.1069, c = 0.8646, and K 1 = 5.3.
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT