Page 303 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 303
QC: —/—
P2: KVU/KXT
P1: KVU/KXT
AT029-Manual-v7.cls
20:46
AT029-Manual
AT029-06
June 22, 2007
Pressure, PSIA
67 8 9 10,000
6 78 9 1,000
4 500
4 50
10 T1: IML 30 6. THERMODYNAMIC RELATIONS FOR PROPERTY ESTIMATIONS 283
300
67 8 9100
4
3,000
2
2
2
1,000
9 9
8 References: 8
7 1. Petrol, Trans. AIME 198 226 (1953) 7
6 6
2. Petrol, Trans. AIME 195 99 (1952)
5 5
3. C.E.P. Phase Equilibria Symposium
4 Vol. 48 No. 2P. 121 (1952) 4
4. I. & E.C. 30 534 (1946)
3 3
2 2
100
9 9
8 8
7 7
6 6
5 5
4 4
3 3
2
2
10 Temperatur, °F
500
9 400 9
8 8
7 300 7
6 6
5 200 5
250
4 150 4
K = y /x 3 60 100 3 K = y /x
2 2
1.0
9 9
8 8
7 7
6 6
5 5
4 4
3 3
2 2
0.1
9 9
8 8
7 7
6 6
5 5
4 4
3 3
2 2
0.01
10 2 30 4 50 67 8 9100 2 300 4 500 6 7 8 9 1,000 2 3,000 4 6 7 8 9 10,000
Hydrogen Sulfide
Pressure, PSIA
CONV. PRESS. 3000 PSIA
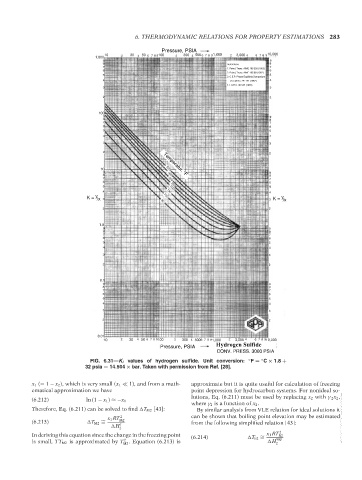
FIG. 6.31—K i values of hydrogen sulfide. Unit conversion: ◦ F = C × 1.8 +
◦
32 psia = 14.504 × bar. Taken with permission from Ref. [28].
x 1 (= 1 − x 2 ), which is very small (x 1 1), and from a math- approximate but it is quite useful for calculation of freezing
ematical approximation we have point depression for hydrocarbon systems. For nonideal so-
lutions, Eq. (6.211) must be used by replacing x 2 with γ 2 x 2 ,
(6.212) ln (1 − x 1) ≈−x 1
where γ 2 is a function of x 2 .
Therefore, Eq. (6.211) can be solved to find T M2 [43]: By similar analysis from VLE relation for ideal solutions it
x 1 RT 2 can be shown that boiling point elevation may be estimated --`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
(6.213) T M2 = M2 from the following simplified relation [43]:
∼
H f
2
2
In deriving this equation since the change in the freezing point (6.214) ∼ x 1 RT B2
2
is small, TT M2 is approximated by T . Equation (6.213) is T b2 = H vap
M2 2
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT