Page 327 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 327
P2: IML/FFX
QC: IML/FFX
P1: IML/FFX
AT029-Manual
AT029-07
June 22, 2007
AT029-Manual-v7.cls
7. APPLICATIONS: ESTIMATION OF THERMOPHYSICAL PROPERTIES 307
100
TECHNICAL DATA BOOK T1: IML 17:40
10 February 1994
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---
1
0.1
0 25 50 75 100 150 200 250 300 400 500
◦
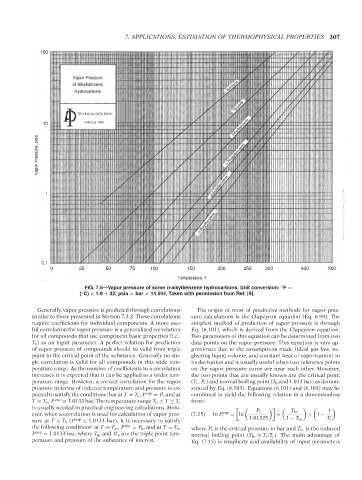
FIG. 7.5—Vapor pressure of some n-alkylbenzene hydrocarbons. Unit conversion: F =
( C) × 1.8 + 32; psia = bar × 14.504. Taken with permission from Ref. [9].
◦
Generally, vapor pressure is predicted through correlations The origin of most of predictive methods for vapor pres-
similar to those presented in Section 7.3.2. These correlations sure calculations is the Clapeyron equation (Eq. 6.99). The
require coefficients for individual components. A more use- simplest method of prediction of vapor pressure is through
ful correlation for vapor pressure is a generalized correlation Eq. (6.101), which is derived from the Clapeyron equation.
for all compounds that use component basic properties (i.e., Two parameters of this equation can be determined from two
T b ) as an input parameter. A perfect relation for prediction data points on the vapor pressure. This equation is very ap-
of vapor pressure of compounds should be valid from triple proximate due to the assumptions made (ideal gas law, ne-
point to the critical point of the substance. Generally no sin- glecting liquid volume, and constant heat of vaporization) in
gle correlation is valid for all compounds in this wide tem- its derivation and is usually useful when two reference points
perature range. As the number of coefficients in a correlation on the vapor pressure curve are near each other. However,
increases it is expected that it can be applied to a wider tem- the two points that are usually known are the critical point
perature range. However, a correct correlation for the vapor (T c , P c ) and normal boiling point (T b and 1.013 bar) as demon-
pressure in terms of reduced temperature and pressure is ex- strated by Eq. (6.103). Equations (6.101) and (6.103) may be
pected to satisfy the conditions that at T = T c , P vap = P c and at combined to yield the following relation in a dimensionless
T = T b , P vap = 1.0133 bar. The temperature range T b ≤ T ≤ T c form:
is usually needed in practical engineering calculations. How-
1
ever, when a correlation is used for calculation of vapor pres- (7.15) ln P r vap = ln P c × T br × 1 −
sure at T ≤ T b (P vap ≤ 1.0133 bar), it is necessary to satisfy 1.01325 1 − T br T r
the following conditions: at T = T tp , P vap = P tp and at T = T b , where P c is the critical pressure in bar and T br is the reduced
P vap = 1.0133 bar, where T tp and P tp are the triple point tem- normal boiling point (T br = T b /T c ). The main advantage of
perature and pressure of the substance of interest. Eq. (7.15) is simplicity and availability of input parameters
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT