Page 331 - Characterization and Properties of Petroleum Fractions - M.R. Riazi
P. 331
T1: IML
QC: IML/FFX
P2: IML/FFX
P1: IML/FFX
AT029-Manual-v7.cls
June 22, 2007
17:40
AT029-07
AT029-Manual
7. APPLICATIONS: ESTIMATION OF THERMOPHYSICAL PROPERTIES 311
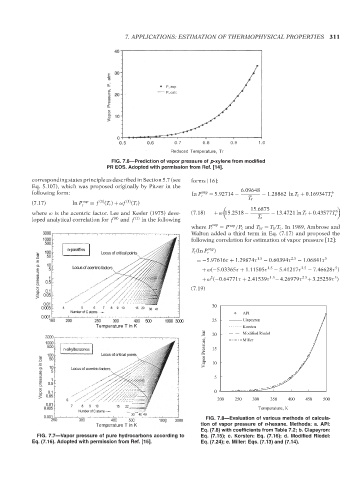
FIG. 7.6—Prediction of vapor pressure of p-xylene from modified
PR EOS. Adopted with permission from Ref. [14].
corresponding states principle as described in Section 5.7 (see forms [16]:
Eq. 5.107), which was proposed originally by Pitzer in the
following form: ln P vap 6.09648 − 1.28862 ln T r + 0.169347T 6
r = 5.92714 − r
T r
(7.17) ln P vap = f (0) (T r ) + ωf (1) (T r )
r
15.6875
where ω is the acentric factor. Lee and Kesler (1975) deve- (7.18) + ω 15.2518 − − 13.4721 ln T r + 0.43577T r 6
loped analytical correlation for f (0) and f (1) in the following T r
vap vap
where P r = P /P c and T br = T b /T c . In 1989, Ambrose and
Walton added a third term in Eq. (7.17) and proposed the
following correlation for estimation of vapor pressure [12]:
T r (ln P r vap )
1.5 2.5 5
=−5.97616τ + 1.29874τ − 0.60394τ − 1.06841τ
1.5 2.5 5
+ ω(−5.03365τ + 1.11505τ − 5.41217τ − 7.46628τ )
2
5
+ ω (−0.64771τ + 2.41539τ 1.5 − 4.26979τ 2.5 + 3.25259τ )
(7.19)
30
API
25 Clapeyron
Temperature T in K 20 Korsten
Vapor Pressure, bar 15 Miller
Modified Riedel
10
5
0
200 250 300 350 400 450 500
Temperature, K
FIG. 7.8—Evaluation of various methods of calcula-
Temperature T in K tion of vapor pressure of n-hexane. Methods: a. API:
Eq. (7.8) with coefficients from Table 7.2; b. Clapeyron:
FIG. 7.7—Vapor pressure of pure hydrocarbons according to Eq. (7.15); c. Korsten: Eq. (7.16); d. Modified Riedel:
Eq. (7.16). Adopted with permission from Ref. [15]. Eq. (7.24); e. Miller: Eqs. (7.13) and (7.14).
Copyright ASTM International
Provided by IHS Markit under license with ASTM Licensee=International Dealers Demo/2222333001, User=Anggiansah, Erick
No reproduction or networking permitted without license from IHS Not for Resale, 08/26/2021 21:56:35 MDT
--`,```,`,``````,`,````,```,,-`-`,,`,,`,`,,`---