Page 856 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 856
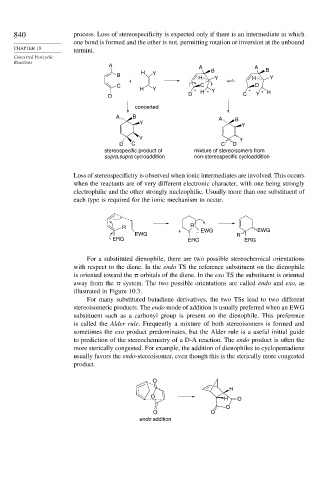
840 process. Loss of stereospecificity is expected only if there is an intermediate in which
one bond is formed and the other is not, permitting rotation or inversion at the unbound
CHAPTER 10
termini.
Concerted Pericyclic
Reactions
A A A
H B B
B Y H Y
+ Y H
C C D
H Y * H * Y * H
D D C Y *
concerted
A B A B
Y Y
Y Y
D C C D
stereospecific product of mixture of stereoisomers from
supra,supra cycloaddition non-stereospecific cycloaddition
Loss of stereospecificity is observed when ionic intermediates are involved. This occurs
when the reactants are of very different electronic character, with one being strongly
electrophilic and the other strongly nucleophilic. Usually more than one substituent of
each type is required for the ionic mechanism to occur.
R R
+ EWG EWG
EWG – R
ERG ERG ERG
For a substituted dienophile, there are two possible stereochemical orientations
with respect to the diene. In the endo TS the reference substituent on the dienophile
is oriented toward the orbitals of the diene. In the exo TS the substituent is oriented
away from the system. The two possible orientations are called endo and exo,as
illustrated in Figure 10.3.
For many substituted butadiene derivatives, the two TSs lead to two different
stereoisomeric products. The endo mode of addition is usually preferred when an EWG
substituent such as a carbonyl group is present on the dienophile. This preference
is called the Alder rule. Frequently a mixture of both stereoisomers is formed and
sometimes the exo product predominates, but the Alder rule is a useful initial guide
to prediction of the stereochemistry of a D-A reaction. The endo product is often the
more sterically congested. For example, the addition of dienophiles to cyclopentadiene
usually favors the endo-stereoisomer, even though this is the sterically more congested
product.
O
H
O
H O
O
O O
endo addition