Page 967 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 967
applied to interpretation of the regiochemistry of 1,3-dipolar cycloaddition reactions. 349 951
The DFT concept of local softness (see Topic 1.5) has been applied to regioselectivity.
PROBLEMS
Chandra and co-workers have emphasized in particular that softness matching may be
a determining factor in regiochemistry. 350 As discussed in connection with the D-A
reaction, the global electrophilicity parameter , as defined in DFT, 341 can provide
some insight into relative reactivity of 1,3-dipoles. Domingo and co-workers have
calculated and N max for representative 1,3-dipoles are given in Table 10.12.
There are some anomalies in Table 10.12; for example, the nitro group is listed as
strongly electrophilic, but in fact is not reactive at all in normal 1,3-dipolar cycloaddi-
tions. The scale is also applicable only to the reactions in which the dipolarophile is
acting as the electrophilic component; that is, in FMO terminology, the LUMO -
dipole
HOMO dipolarophile interaction is dominant.
Problems
(References for these problems will be found on page 1165.)
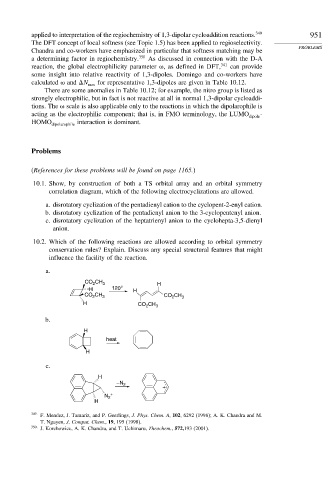
10.1. Show, by construction of both a TS orbital array and an orbital symmetry
correlation diagram, which of the following electrocyclizations are allowed.
a. disrotatory cyclization of the pentadienyl cation to the cyclopent-2-enyl cation.
b. disrotatory cyclization of the pentadienyl anion to the 3-cyclopentenyl anion.
c. disrotatory cyclization of the heptatrienyl anion to the cyclohepta-3,5-dienyl
anion.
10.2. Which of the following reactions are allowed according to orbital symmetry
conservation rules? Explain. Discuss any special structural features that might
influence the facility of the reaction.
a.
CO CH 3 H
2
H 120° H
CO CH 3 CO CH 3
2
2
H CO CH 3
2
b.
H
heat
H
c.
H
–N 2
+
N 2 +
H
349 F. Mendez, J. Tamariz, and P. Geerlings, J. Phys. Chem. A, 102, 6292 (1998); A. K. Chandra and M.
T. Nguyen, J. Comput. Chem., 19, 195 (1998).
350
J. Korchowiec, A. K. Chandra, and T. Uchimaru, Theochem., 572,193 (2001).