Page 970 - Advanced Organic Chemistry Part A - Structure and Mechanisms, 5th ed (2007) - Carey _ Sundberg
P. 970
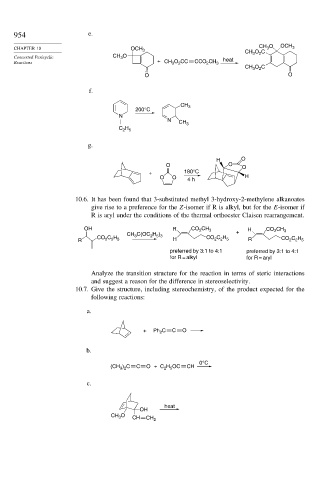
954 e.
O OCH 3
CHAPTER 10 OCH 3 CH O C
CH 3
Concerted Pericyclic CH O heat 3 2
3
Reactions + CH O CC CCO CH 3
2
3
2
CH 3 O C
2
O O
f.
CH 3
200°C
N
N
CH 3
C H
2 5
g.
H O
O O O
+ 180°C
O O 4 h H
10.6. It has been found that 3-substituted methyl 3-hydroxy-2-methylene alkanoates
give rise to a preference for the Z-isomer if R is alkyl, but for the E-isomer if
R is aryl under the conditions of the thermal orthoester Claisen rearrangement.
OH R CO CH 3 H CO CH 3
2
2
CH C(OC H ) +
CO C H 3 2 5 3 CO C H CO C H
R 2 2 5 H 2 2 5 R 2 2 5
preferred by 3:1 to 4:1 preferred by 3:1 to 4:1
for R = alkyl for R = aryl
Analyze the transition structure for the reaction in terms of steric interactions
and suggest a reason for the difference in stereoselectivity.
10.7. Give the structure, including stereochemistry, of the product expected for the
following reactions:
a.
+ Ph C C O
2
b.
0°C
(CH ) C C O + C H OC CH
3 2
2 5
c.
heat
OH
CH O CH CH 2
3