Page 100 - Advances in Biomechanics and Tissue Regeneration
P. 100
96 6. REVIEW OF THE ESSENTIAL ROLES OF SMCS IN ATAA BIOMECHANICS
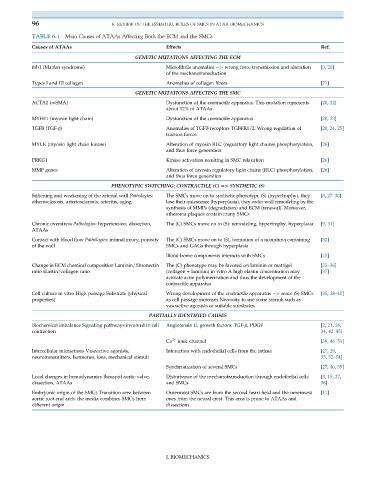
TABLE 6.1 Main Causes of ATAAs Affecting Both the ECM and the SMCs
Causes of ATAAs Effects Ref.
GENETIC MUTATIONS AFFECTING THE ECM
fnb1 (Marfan syndrome) Microfibrils anomalies ¼> wrong force transmission and alteration [3, 20]
of the mechanotransduction
Types I and III collagen Anomalies of collagen fibers [21]
GENETIC MUTATIONS AFFECTING THE SMC
ACTA2 (α-SMA) Dysfunction of the contractile apparatus. This mutation represents [20, 22]
about 12% of ATAAs
MYH11 (myosin light chain) Dysfunction of the contractile apparatus [20, 23]
TGFB (TGF-β) Anomalies of TGFB receptors TGBFR1/2. Wrong regulation of [20, 24, 25]
traction forces
MYLK (myosin light chain kinase) Alteration of myosin RLC (regulatory light chains) phosphorylation, [26]
and thus force generation
PRKG1 Kinase activation resulting in SMC relaxation [26]
MMP genes Alteration of myosin regulatory light chains (RLC) phosphorylation, [26]
and thus force generation
PHENOTYPIC SWITCHING: CONTRACTILE (C) 5> SYNTHETIC (S)
Stiffening and weakening of the arterial wall Pathologies: The SMCs move on to synthetic phenotype (S) (hypertrophy), they [3, 27–30]
atherosclerosis, arteriosclerosis, arteritis, aging lose their quiescence (hyperplasia), they order wall remodeling by the
synthesis of MMPs (degradation) and ECM (renewal). Moreover,
atheroma plaques contain many SMCs
Chronic overstress Pathologies: hypertension, dissection, The (C) SMCs move on to (S): remodeling, hypertrophy, hyperplasia [9, 31]
ATAAs
Contact with blood flow Pathologies: intimal injury, porosity The (C) SMCs move on to (S), formation of a neointima containing [32]
of the wall SMCs and GAGs through hyperplasia
Blood-borne components interacts with SMCs [15]
Change in ECM chemical composition Laminin/fibronectin The (C) phenotype may be favored on laminin or matrigel [33–36]
ratio Elastin/collagen ratio (collagen + laminin) in vitro A high elastin concentration may [37]
activate actin polymerization and thus the development of the
contractile apparatus
Cell culture in vitro High passage Substrate (physical Wrong development of the contractile apparatus ¼> more (S) SMCs [35, 38–41]
properties) as cell passage increases Necessity to use some stimuli such as
vasoactive agonists or suitable substrates
PARTIALLY IDENTIFIED CAUSES
Biochemical imbalance Signaling pathways involved in cell Angiotensin II, growth factors: TGF-β, PDGF [2, 21, 26,
contraction 34, 42–45]
Ca 2+ ionic channel [39, 46–51]
Intercellular interactions Vasoactive agonists, Interaction with endothelial cells from the intima [27, 29,
neurotransmitters, hormones, ions, mechanical stimuli 35, 52–54]
Synchronization of several SMCs [27, 46, 55]
Local changes in hemodynamics Bicuspid aortic valve, Disturbance of the mechanotransduction through endothelial cells [3, 15, 27,
dissection, ATAAs and SMCs 56]
Embryonic origin of the SMCs Transition area between Outermost SMCs are from the second heart field and the innermost [11]
aortic root and arch: the media combines SMCs from ones from the neural crest. This area is prone to ATAAs and
different origin dissections
I. BIOMECHANICS