Page 101 - Advances in Biomechanics and Tissue Regeneration
P. 101
6.2 BASICS OF AORTIC WALL MECHANICS AND PASSIVE BIOMECHANICAL ROLE OF SMCS 97
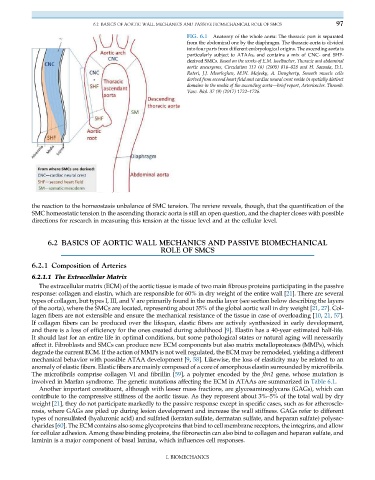
FIG. 6.1 Anatomy of the whole aorta: The thoracic part is separated
from the abdominal one by the diaphragm. The thoracic aorta is divided
into four parts from different embryological origins. The ascending aorta is
particularly subject to ATAAs, and contains a mix of CNC- and SHF-
derived SMCs. Based on the works of E.M. Isselbacher, Thoracic and abdominal
aortic aneurysms, Circulation 111 (6) (2005) 816–828 and H. Sawada, D.L.
Rateri, J.J. Moorleghen, M.W. Majesky, A. Daugherty, Smooth muscle cells
derived from second heart field and cardiac neural crest reside in spatially distinct
domains in the media of the ascending aorta—brief report, Arterioscler. Thromb.
Vasc. Biol. 37 (9) (2017) 1722–1726.
the reaction to the homeostasis unbalance of SMC tension. The review reveals, though, that the quantification of the
SMC homeostatic tension in the ascending thoracic aorta is still an open question, and the chapter closes with possible
directions for research in measuring this tension at the tissue level and at the cellular level.
6.2 BASICS OF AORTIC WALL MECHANICS AND PASSIVE BIOMECHANICAL
ROLE OF SMCS
6.2.1 Composition of Arteries
6.2.1.1 The Extracellular Matrix
The extracellular matrix (ECM) of the aortic tissue is made of two main fibrous proteins participating in the passive
response: collagen and elastin, which are responsible for 60% in dry weight of the entire wall [21]. There are several
types of collagen, but types I, III, and V are primarily found in the media layer (see section below describing the layers
of the aorta), where the SMCs are located, representing about 35% of the global aortic wall in dry weight [21, 27]. Col-
lagen fibers are not extensible and ensure the mechanical resistance of the tissue in case of overloading [10, 21, 57].
If collagen fibers can be produced over the lifespan, elastic fibers are actively synthesized in early development,
and there is a loss of efficiency for the ones created during adulthood [9]. Elastin has a 40-year estimated half-life.
It should last for an entire life in optimal conditions, but some pathological states or natural aging will necessarily
affect it. Fibroblasts and SMCs can produce new ECM components but also matrix metalloproteases (MMPs), which
degrade the current ECM. If the action of MMPs is not well regulated, the ECM may be remodeled, yielding a different
mechanical behavior with possible ATAA development [9, 58]. Likewise, the loss of elasticity may be related to an
anomaly of elastic fibers. Elastic fibers are mainly composed of a core of amorphous elastin surrounded by microfibrils.
The microfibrils comprise collagen VI and fibrillin [59], a polymer encoded by the fbn1 gene, whose mutation is
involved in Marfan syndrome. The genetic mutations affecting the ECM in ATAAs are summarized in Table 6.1.
Another important constituent, although with lesser mass fractions, are glycosaminoglycans (GAGs), which can
contribute to the compressive stiffness of the aortic tissue. As they represent about 3%–5% of the total wall by dry
weight [21], they do not participate markedly to the passive response except in specific cases, such as for atheroscle-
rosis, where GAGs are piled up during lesion development and increase the wall stiffness. GAGs refer to different
types of nonsulfated (hyaluronic acid) and sulfated (keratan sulfate, dermatan sulfate, and heparan sulfate) polysac-
charides [60]. The ECM contains also some glycoproteins that bind to cell membrane receptors, the integrins, and allow
for cellular adhesion. Among these binding proteins, the fibronectin can also bind to collagen and heparan sulfate, and
laminin is a major component of basal lamina, which influences cell responses.
I. BIOMECHANICS