Page 137 - Advances in Biomechanics and Tissue Regeneration
P. 137
7.3 VULNERABILITY IN REGIONALLY ISCHEMIC HUMAN HEART: EFFECT OF THE EXTRACELLULAR POTASSIUM CONCENTRATION 133
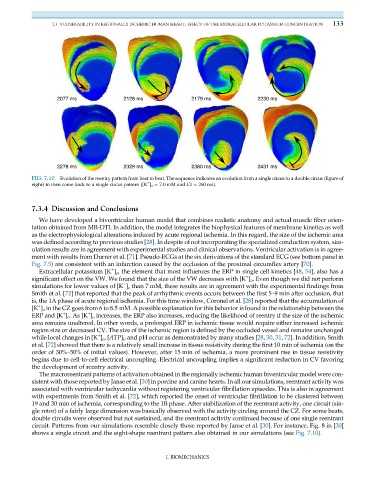
FIG. 7.10 Evolution of the reentry pattern from beat to beat. The sequence indicates an evolution from a single circus to a double circus (figure of
+
eight) to then come back to a single circus pattern ([K ] o ¼ 7.0 mM and CI ¼ 260 ms).
7.3.4 Discussion and Conclusions
We have developed a biventricular human model that combines realistic anatomy and actual muscle fiber orien-
tation obtained from MR-DTI. In addition, the model integrates the biophysical features of membrane kinetics as well
as the electrophysiological alterations induced by acute regional ischemia. In this regard, the size of the ischemic area
was defined according to previous studies [28]. In despite of not incorporating the specialized conduction system, sim-
ulation results are in agreement with experimental studies and clinical observations. Ventricular activation is in agree-
ment with results from Durrer et al. [71]. Pseudo-ECGs at the six derivations of the standard ECG (see bottom panel in
Fig. 7.5) are consistent with an infarction caused by the occlusion of the proximal circumflex artery [70].
+
Extracellular potassium [K ] o , the element that most influences the ERP in single cell kinetics [48, 54], also has a
+
significant effect on the VW. We found that the size of the VW decreases with [K ] o . Even though we did not perform
+
simulations for lower values of [K ] o than 7 mM, these results are in agreement with the experimental findings from
Smith et al. [72] that reported that the peak of arrhythmic events occurs between the first 5–9 min after occlusion, that
is, the 1A phase of acute regional ischemia. For this time window, Coronel et al. [28] reported that the accumulation of
+
[K ] o in the CZ goes from 6 to 8.5 mM. A possible explanation for this behavior is found in the relationship between the
+
+
ERP and [K ] o .As[K ] o increases, the ERP also increases, reducing the likelihood of reentry if the size of the ischemic
area remains unaltered. In other words, a prolonged ERP in ischemic tissue would require either increased ischemic
region size or decreased CV. The size of the ischemic region is defined by the occluded vessel and remains unchanged
+
while local changes in [K ] o ,[ATP] i , and pH occur as demonstrated by many studies [28, 30, 31, 72]. In addition, Smith
et al. [72] showed that there is a relatively small increase in tissue resistivity during the first 10 min of ischemia (on the
order of 30%–50% of initial values). However, after 15 min of ischemia, a more prominent rise in tissue resistivity
begins due to cell-to-cell electrical uncoupling. Electrical uncoupling implies a significant reduction in CV favoring
the development of reentry activity.
The macroreentrant patterns of activation obtained in the regionally ischemic human biventricular model were con-
sistent with those reported by Janse et al. [30] in porcine and canine hearts. In all our simulations, reentrant activity was
associated with ventricular tachycardia without registering ventricular fibrillation episodes. This is also in agreement
with experiments from Smith et al. [72], which reported the onset of ventricular fibrillation to be clustered between
19 and 30 min of ischemia, corresponding to the 1B phase. After stabilization of the reentrant activity, one circuit (sin-
gle rotor) of a fairly large dimension was basically observed with the activity circling around the CZ. For some beats,
double circuits were observed but not sustained, and the reentrant activity continued because of one single reentrant
circuit. Patterns from our simulations resemble closely those reported by Janse et al. [30]. For instance, Fig. 8 in [30]
shows a single circuit and the eight-shape reentrant pattern also obtained in our simulations (see Fig. 7.10).
I. BIOMECHANICS