Page 40 - Advances in Renewable Energies and Power Technologies
P. 40
2. Properties of Semiconductors for Solar Cells 13
p ffiffiffiffiffiffiffiffiffiffiffi
where Z wa is the wave impedance of the air Z wa ¼ m . It is assumed that
o= ε o
light is falling perpendicular to the surface. For Si, with ε ¼ 12ε o and m ¼ m o the
reflectance is r ¼ 0.3. This means that the reflected part of incident light amounts
to 0.3, which is an appreciable part.
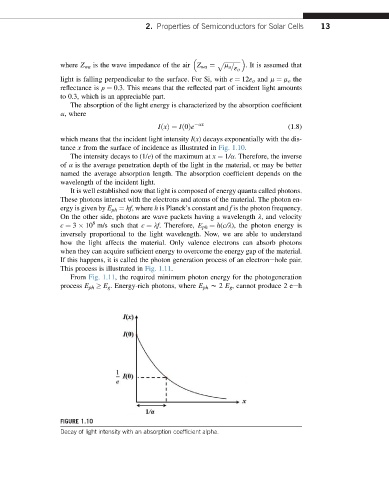
The absorption of the light energy is characterized by the absorption coefficient
a, where
ax
IðxÞ¼ Ið0Þe (1.8)
which means that the incident light intensity I(x) decays exponentially with the dis-
tance x from the surface of incidence as illustrated in Fig. 1.10.
The intensity decays to (1/e) of the maximum at x ¼ 1/a. Therefore, the inverse
of a is the average penetration depth of the light in the material, or may be better
named the average absorption length. The absorption coefficient depends on the
wavelength of the incident light.
It is well established now that light is composed of energy quanta called photons.
These photons interact with the electrons and atoms of the material. The photon en-
ergy is given by E ph ¼ hf, where h is Planck’s constant and f is the photon frequency.
On the other side, photons are wave packets having a wavelength l, and velocity
8
c ¼ 3 10 m/s such that c ¼ lf. Therefore, E ph ¼ h(c/l), the photon energy is
inversely proportional to the light wavelength. Now, we are able to understand
how the light affects the material. Only valence electrons can absorb photons
when they can acquire sufficient energy to overcome the energy gap of the material.
If this happens, it is called the photon generation process of an electronehole pair.
This process is illustrated in Fig. 1.11.
From Fig. 1.11, the required minimum photon energy for the photogeneration
process E ph E g . Energy-rich photons, where E ph w 2 E g , cannot produce 2 eeh
FIGURE 1.10
Decay of light intensity with an absorption coefficient alpha.