Page 80 - Artificial Intelligence for Computational Modeling of the Heart
P. 80
50 Chapter 2 Implementation of a patient-specific cardiac model
2.1.4 Torso modeling
Patient-specific torso geometry can be either segmented auto-
matically from images [202] or, when the torso is not fully visible
in the images, estimated from an atlas by manually registering the
model to the patient. If the images do not have the sufficient field
of view to align the torso, or are not at all available such as in the
case of ultrasound-based workflows, the relative position of the
heart in the thoracic cavity is used as reference for the alignment.
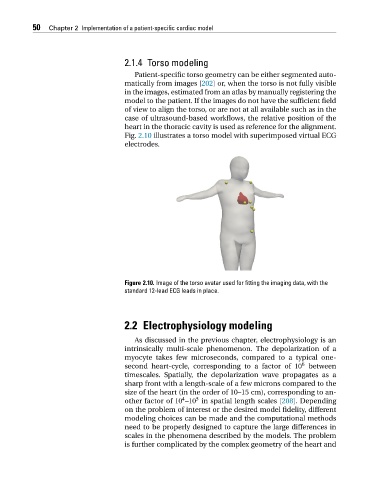
Fig. 2.10 illustrates a torso model with superimposed virtual ECG
electrodes.
Figure 2.10. Image of the torso avatar used for fitting the imaging data, with the
standard 12-lead ECG leads in place.
2.2 Electrophysiology modeling
As discussed in the previous chapter, electrophysiology is an
intrinsically multi-scale phenomenon. The depolarization of a
myocyte takes few microseconds, compared to a typical one-
6
second heart-cycle, corresponding to a factor of 10 between
timescales. Spatially, the depolarization wave propagates as a
sharp front with a length-scale of a few microns compared to the
size of the heart (in the order of 10–15 cm), corresponding to an-
5
4
other factor of 10 –10 in spatial length scales [208]. Depending
on the problem of interest or the desired model fidelity, different
modeling choices can be made and the computational methods
need to be properly designed to capture the large differences in
scales in the phenomena described by the models. The problem
is further complicated by the complex geometry of the heart and