Page 287 - Battery Reference Book
P. 287
24/10 Lithium batteries
(i) by the introduction of solid state electronics into Ampoule
battery design, and Glass-to-metal Support
(ii) by thermal management of the cells using
circulating electrolytes, phase change heat sinks
and cooling fins.
24.3 Lithium-vanadium pentoxide
primary batteries
The lithium-vanadium pentoxide cell is very attractive
(as is the lithium-manganese dioxide cell) because the
cathode, vanadium pentoxide, with its high oxidation
state can provide a high open circuit voltage (3.4
V). It has a high energy density (224Whkg-') and
power density. The cell exhibits multiple voltages on
discharge under a 1000 R load giving four plateaux at
3.4, 3.2, 2.4 and 2.0V and under 3.3 K R load giving
two plateaux at 3.0 and 1.8 V.
A disadvantage of the vanadium pentoxide cathode
is its relatively low electronic conductivity. To offset
this the cell is modified with 10% wlw carbon powder
and 5% PTFE binder. The anode is pure lithium. The
electrolyte consists of IM lithium perchlorate dissolved --- --
in propylene carbonate or 1:l propylene carbonate: 1:2
dimethoxyethane.
Lithium-vanadium pentoxide reserve cells are
available in the capacity range 100-5oOmA. They
undergo no capacity loss during 10 years' storage.
A major outlet is munition system batteries. The
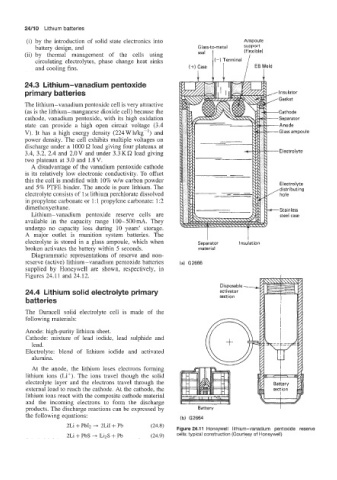
electrolyte is stored in a glass ampoule, which when Separator I mulation
broken activates the battery within 5 seconds. material
Diagrammatic representations of reserve and non-
reserve (active) lithium-vanadium pentoxide batteries (a) G2666
supplied by Honeywell are shown, respectively, in
Figures 24.11 and 24.12.
24.4 Lithium solid electrolyte primary
batteries
The Duracell solid electrolyte cell is made of the
following materials:
Anode: high-purity lithium sheet.
Cathode: mixture of lead iodide, lead sulphide and
lead.
Electrolyte: blend of lithium iodide and activated
alumina.
At the anode, the lithium loses electrons forming
lithium ions (Lif). The ions travel though the solid
electrolyte layer and the electrons travel through the
external load to reach the cathode. At the cathode, the
lithium ions react with the composite cathode material
and the incoming electrons to form the discharge
products. The discharge reactions can be expressed by Battery I
the following equations: (b) G2664
2Li + PbI2 + 2LiI + Pb (24.8)
Figure 24.1 1 Honeywell lithium-vanadium pentoxide reserve
2Li + PbS + LizS + Pb (24.9) cells: typical construction (Courtesy of Honeywell)