Page 399 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 399
17.2 A Generic Strategy for the Synthesis of Sialoconjugate Libraries 375
Phe192
Ser82
Phe193
Tyr179
Gln104
Gly176
Asn175 Thr106
Lys142
Asp209
Arg173 Arg165
Asp211
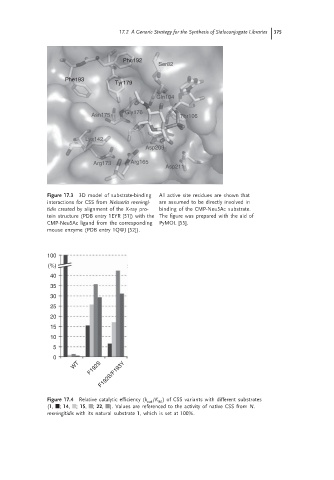
Figure 17.3 3D model of substrate-binding All active site residues are shown that
interactions for CSS from Neisseria meningi- are assumed to be directly involved in
tidis created by alignment of the X-ray pro- binding of the CMP-Neu5Ac substrate.
tein structure (PDB entry 1EYR [51]) with the The figure was prepared with the aid of
CMP-Neu5Ac ligand from the corresponding PyMOL [55].
mouse enzyme (PDB entry 1QWJ [52]).
100
(%)
40
35
30
25
20
15
10
5
0
WT F192S F192S/F193Y
Figure 17.4 Relative catalytic efficiency (k /K ) of CSS variants with different substrates
cat M
(1, ; 14, ; 15, ; 22, ). Values are referenced to the activity of native CSS from N.
meningitidis with its natural substrate 1, which is set at 100%.