Page 398 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 398
374 17 Enzymatic Generation of Sialoconjugate Diversity
NH
NH 2 2
O O O N O N
− O P O P O P O N CSS Sia O P O N
O – O – O – O O Mg 2+ O – O O
CTP HO OH Sia-OH CMP-Sia HO OH
O O O O
− − –
O P O P OH O P O P O
O – O – O – O –
O H + O
− O 3 S − O 3 S
OH O –
Yellow Purple-red
2.0 < pH < 7.2 pH > 8.8
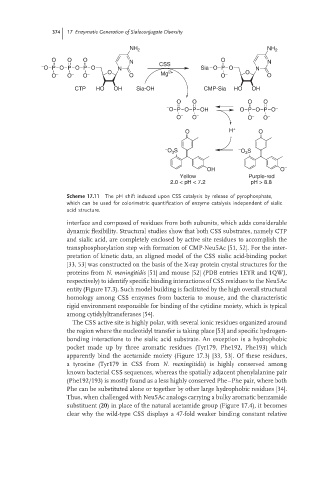
Scheme 17.11 The pH shift induced upon CSS catalysis by release of pyrophosphate,
which can be used for colorimetric quantification of enzyme catalysis independent of sialic
acid structure.
interface and composed of residues from both subunits, which adds considerable
dynamic flexibility. Structural studies show that both CSS substrates, namely CTP
and sialic acid, are completely enclosed by active site residues to accomplish the
transphosphorylation step with formation of CMP-Neu5Ac [51, 52]. For the inter-
pretation of kinetic data, an aligned model of the CSS sialic acid-binding pocket
[33, 53] was constructed on the basis of the X-ray protein crystal structures for the
proteins from N. meningitidis [51] and mouse [52] (PDB entries 1EYR and 1QWJ,
respectively) to identify specific binding interactions of CSS residues to the Neu5Ac
entity (Figure 17.3). Such model building is facilitated by the high overall structural
homology among CSS enzymes from bacteria to mouse, and the characteristic
rigid environment responsible for binding of the cytidine moiety, which is typical
among cytidylyltransferases [54].
The CSS active site is highly polar, with several ionic residues organized around
the region where the nucleotidyl transfer is taking place [53] and specific hydrogen-
bonding interactions to the sialic acid substrate. An exception is a hydrophobic
pocket made up by three aromatic residues (Tyr179, Phe192, Phe193) which
apparently bind the acetamide moiety (Figure 17.3) [33, 53]. Of these residues,
a tyrosine (Tyr179 in CSS from N. meningitidis) is highly conserved among
known bacterial CSS sequences, whereas the spatially adjacent phenylalanine pair
(Phe192/193) is mostly found as a less highly conserved Phe–Phe pair, where both
Phe can be substituted alone or together by other large hydrophobic residues [34].
Thus, when challenged with Neu5Ac analogs carrying a bulky aromatic benzamide
substituent (20) in place of the natural acetamide group (Figure 17.4), it becomes
clear why the wild-type CSS displays a 47-fold weaker binding constant relative