Page 394 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 394
370 17 Enzymatic Generation of Sialoconjugate Diversity
OH
X Y OH
Y HN NeuA
HO O NH O CO 2 H
HO OH Pyruvate X HO OH
OH OH OH OH
HO OH HO OH HO OH HO OH
NH O CO 2 H NH O CO H NH O CO H NH O CO H
2
2
2
HO OH HO OH HO OH HO OH
O 9 O 10 O 11 O 12
OH OH OH
HO OH HO OH HO OH
O H O CO H O CO H
NH CO 2 NH 2 N 3 NH 2
HO OH HO OH HO OH
O 13 O 14 O 15
OH OH OH
HO OH HO OH HO OH
O NH O CO 2 H O NH O CO H O NH O CO 2 H
2
HO OH HO OH HO OH
O 16 O 17 O 18
OH OH OH
HO OH HO OH HO OH
O CO H O H O CO H
O NH 2 NH CO 2 NH 2
HO OH HO OH HO OH
O 19 O 20 O 21
OH OH OH
HO OH HO OH HO OH
NH O CO 2 H O NH O CO 2 H F 3 C NH O CO 2 H
O HO OH HO OH HO OH
O 22 23 O 24
NHBoc
O
OH OH O OH
HO OH HO OH N O OH
H
NH O CO H NH O CO 2 H O AcHN O CO H
2
+ S 2
H 3 N HO OH HO OH HO OH
O 25 O O 26 27
H OH H OH C F 13 O OH
6
N OH N OH O OH
2
O AcNH O CO H O AcNH O CO H AcHN O CO H
2
2
HO OH HO OH HO OH
28 29 30
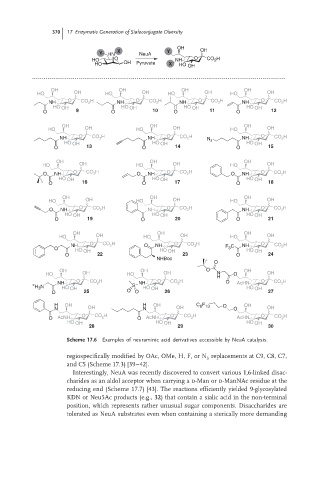
Scheme 17.6 Examples of neuraminic acid derivatives accessible by NeuA catalysis.
regiospecifically modified by OAc, OMe, H, F, or N replacements at C9, C8, C7,
3
and C5 (Scheme 17.3) [39–42].
Interestingly, NeuA was recently discovered to convert various 1,6-linked disac-
charides as an aldol acceptor when carrying a d-Man or d-ManNAc residue at the
reducing end (Scheme 17.7) [43]. The reactions efficiently yielded 9-glycosylated
KDN or Neu5Ac products (e.g., 32)thatcontainasialicacidinthe non-terminal
position, which represents rather unusual sugar components. Disaccharides are
tolerated as NeuA substrates even when containing a sterically more demanding