Page 397 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 397
17.2 A Generic Strategy for the Synthesis of Sialoconjugate Libraries 373
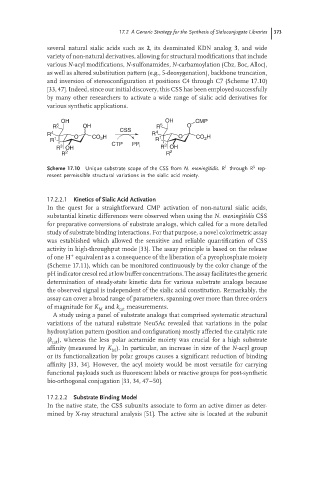
several natural sialic acids such as 2, its deaminated KDN analog 3, and wide
variety of non-natural derivatives, allowing for structural modifications that include
various N-acyl modifications, N-sulfonamides, N-carbamoylation (Cbz, Boc, Alloc),
as well as altered substitution pattern (e.g., 5-deoxygenation), backbone truncation,
and inversion of stereoconfiguration at positions C4 through C7 (Scheme 17.10)
[33, 47]. Indeed, since our initial discovery, this CSS has been employed successfully
by many other researchers to activate a wide range of sialic acid derivatives for
various synthetic applications.
OH OH CMP
R 5 OH CSS R 5 O
R 4 O CO H R 4 O CO H
R 1 2 R 1 2
R 3 OH CTP PP i R 3 OH
R 2 R 2
5
1
Scheme 17.10 Unique substrate scope of the CSS from N. meningitidis.R through R rep-
resent permissible structural variations in the sialic acid moiety.
17.2.2.1 Kinetics of Sialic Acid Activation
In the quest for a straightforward CMP activation of non-natural sialic acids,
substantial kinetic differences were observed when using the N. meningitidis CSS
for preparative conversions of substrate analogs, which called for a more detailed
study of substrate binding interactions. For that purpose, a novel colorimetric assay
was established which allowed the sensitive and reliable quantification of CSS
activity in high-throughput mode [33]. The assay principle is based on the release
+
of one H equivalent as a consequence of the liberation of a pyrophosphate moiety
(Scheme 17.11), which can be monitored continuously by the color change of the
pH indicator cresol red at low buffer concentrations. The assay facilitates the generic
determination of steady-state kinetic data for various substrate analogs because
the observed signal is independent of the sialic acid constitution. Remarkably, the
assay can cover a broad range of parameters, spanning over more than three orders
of magnitude for K and k measurements.
M cat
A study using a panel of substrate analogs that comprised systematic structural
variations of the natural substrate Neu5Ac revealed that variations in the polar
hydroxylation pattern (position and configuration) mostly affected the catalytic rate
(k ), whereas the less polar acetamide moiety was crucial for a high substrate
cat
affinity (measured by K ). In particular, an increase in size of the N-acyl group
M
or its functionalization by polar groups causes a significant reduction of binding
affinity [33, 34]. However, the acyl moiety would be most versatile for carrying
functional payloads such as fluorescent labels or reactive groups for post-synthetic
bio-orthogonal conjugation [33, 34, 47–50].
17.2.2.2 Substrate Binding Model
In the native state, the CSS subunits associate to form an active dimer as deter-
mined by X-ray structural analysis [51]. The active site is located at the subunit