Page 393 - Cascade_Biocatalysis_Integrating_Stereoselective_and_Environmentally_Friendly_Reactions
P. 393
17.2 A Generic Strategy for the Synthesis of Sialoconjugate Libraries 369
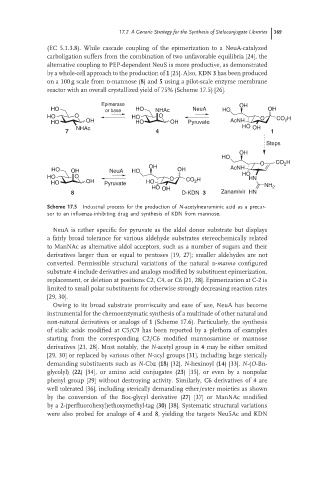
(EC 5.1.3.8). While cascade coupling of the epimerization to a NeuA-catalyzed
carboligation suffers from the combination of two unfavorable equilibria [24], the
alternative coupling to PEP-dependent NeuS is more productive, as demonstrated
by a whole-cell approach to the production of 1 [25]. Also, KDN 3 has been produced
on a 100 g scale from d-mannose (8)and 5 using a pilot-scale enzyme membrane
reactor with an overall crystallized yield of 75% (Scheme 17.5) [26].
Epimerase OH
HO or base HO NHAc NeuA HO OH
HO O HO O O CO 2 H
HO OH HO OH Pyruvate AcNH
NHAc HO OH
7 4 1
Steps
OH
HO
O CO 2 H
OH AcNH
HO OH NeuA HO OH
HO O O CO H HO HN
HO OH Pyruvate HO 2
HO OH NH 2
8 D-KDN 3 Zanamivir HN
Scheme 17.5 Industrial process for the production of N-acetylneuraminic acid as a precur-
sor to an influenza-inhibiting drug and synthesis of KDN from mannose.
NeuA is rather specific for pyruvate as the aldol donor substrate but displays
a fairly broad tolerance for various aldehyde substrates stereochemically related
to ManNAc as alternative aldol acceptors, such as a number of sugars and their
derivatives larger than or equal to pentoses [19, 27]; smaller aldehydes are not
converted. Permissible structural variations of the natural d-manno configured
substrate 4 include derivatives and analogs modified by substituent epimerization,
replacement, or deletion at positions C2, C4, or C6 [21, 28]. Epimerization at C-2 is
limited to small polar substituents for otherwise strongly decreasing reaction rates
[29, 30].
Owing to its broad substrate promiscuity and ease of use, NeuA has become
instrumental for the chemoenzymatic synthesis of a multitude of other natural and
non-natural derivatives or analogs of 1 (Scheme 17.6). Particularly, the synthesis
of sialic acids modified at C5/C9 has been reported by a plethora of examples
starting from the corresponding C2/C6 modified mannosamine or mannose
derivatives [21, 28]. Most notably, the N-acetyl group in 4 may be either omitted
[29, 30] or replaced by various other N-acyl groups [31], including large sterically
demanding substituents such as N-Cbz (18) [32], N-hexinoyl (14) [33], N-(O-Bn-
glycolyl) (22) [34], or amino acid conjugates (23) [35], or even by a nonpolar
phenyl group [29] without destroying activity. Similarly, C6 derivatives of 4 are
well tolerated [36], including sterically demanding ether/ester moieties as shown
by the conversion of the Boc-glycyl derivative (27) [37] or ManNAc modified
by a 2-(perfluorohexyl)ethoxymethyl-tag (30) [38]. Systematic structural variations
were also probed for analogs of 4 and 8, yielding the targets Neu5Ac and KDN