Page 427 - Corrosion Engineering Principles and Practice
P. 427
396 C h a p t e r 1 0 C o r r o s i o n i n S o i l s a n d M i c r o b i o l o g i c a l l y I n f l u e n c e d C o r r o s i o n 397
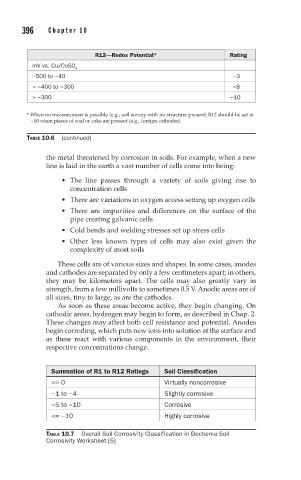
R12—Redox Potential* Rating
mV vs. Cu/CuSO
4
−500 to −40 −3
> −400 to −300 −8
> −300 −10
* When no measurement is possible (e.g., soil survey with no structure present) R12 should be set at
−10 when pieces of coal or coke are present (e.g., foreign cathodes).
TABLE 10.6 (continued)
the metal threatened by corrosion in soils. For example, when a new
line is laid in the earth a vast number of cells come into being:
• The line passes through a variety of soils giving rise to
concentration cells
• There are variations in oxygen access setting up oxygen cells
• There are impurities and differences on the surface of the
pipe creating galvanic cells
• Cold bends and welding stresses set up stress cells
• Other less known types of cells may also exist given the
complexity of most soils
These cells are of various sizes and shapes. In some cases, anodes
and cathodes are separated by only a few centimeters apart; in others,
they may be kilometers apart. The cells may also greatly vary in
strength, from a few millivolts to sometimes 0.5 V. Anodic areas are of
all sizes, tiny to large, as are the cathodes.
As soon as these areas become active, they begin changing. On
cathodic areas, hydrogen may begin to form, as described in Chap. 2.
These changes may affect both cell resistance and potential. Anodes
begin corroding, which puts new ions into solution at the surface and
as these react with various components in the environment, their
respective concentrations change.
Summation of R1 to R12 Ratings Soil Classification
>= 0 Virtually noncorrosive
−1 to −4 Slightly corrosive
−5 to −10 Corrosive
<= −10 Highly corrosive
TABLE 10.7 Overall Soil Corrosivity Classification in Dechema Soil
Corrosivity Worksheet [5]