Page 355 - Earth's Climate Past and Future
P. 355
CHAPTER 18 • Causes of Warming over the Last 125 Years 331
Methane added to the atmosphere comes from Ozone (O ) occurs naturally in the stratosphere, with
3
sources rich in organic carbon but lacking in oxygen: the largest concentrations between 15 and 30 km. Incom-
swampy bogs where plants decay, cattle and other ing ultraviolet (UV) radiation from the Sun liberates indi-
grazing and browsing animals that digest vegetation, vidual O atoms from oxygen (O ) and produces ozone:
2
animal and human waste, and burning of grassy vegeta-
tion. In the absence of oxygen, bacteria break down the Radiation + O → O + O
2
vegetation and extract the carbon, which combines with (UV)
hydrogen to form methane gas. O + O → O 3
2
Increased emissions of CH during the last 200
4
years have resulted from the explosion of populations 0
of humans and livestock. Today the amount of CH
4
produced by human activity is more than twice as large –10
as that from natural sources (see Table 17–1). Ever-
increasing areas of tropical land have been put into rice –20
paddy cultivation in Southeast Asia, and these artificial Ozone change (% vs. 1960 peak) –30
wetlands produce methane. The growing numbers of
cattle and other livestock have increased the amount of –40
methane gas sent to the atmosphere.
Methane also acts as a greenhouse gas by trapping –50
outgoing radiation from the Earth’s surface. Although
its concentration is much lower than that of CO , it is 1960 1970 1980 1990
2 Year
far more effective on a molecule-by-molecule basis in A Antarctic ozone concentration
trapping radiation than CO . The enormous increase in
2
methane concentration during the last two centuries
has also caused the planet to warm. Based on the
observed increases during the last 200 years, methane
has accounted for ~16% of the total greenhouse-gas
effect during the industrial era, although its rate of
increase has slowed since the late 1990s.
18-6 Increases in Chlorofluorocarbons
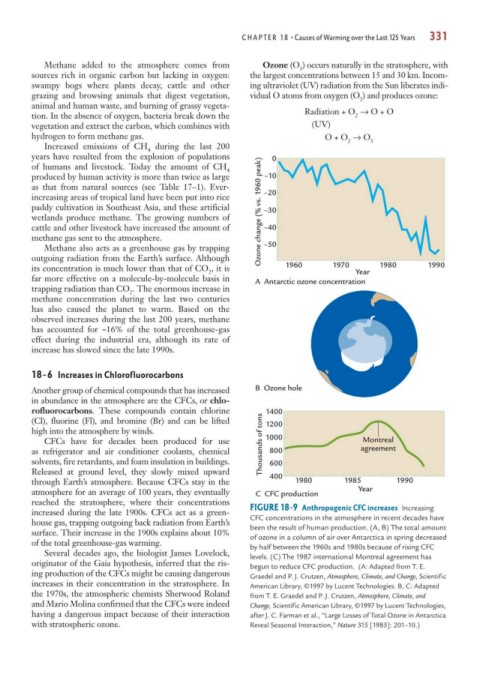
Another group of chemical compounds that has increased B Ozone hole
in abundance in the atmosphere are the CFCs, or chlo-
rofluorocarbons. These compounds contain chlorine
(Cl), fluorine (Fl), and bromine (Br) and can be lifted 1200
high into the atmosphere by winds.
1000
CFCs have for decades been produced for use Thousands of tons 1400 Montreal
as refrigerator and air conditioner coolants, chemical 800 agreement
solvents, fire retardants, and foam insulation in buildings. 600
Released at ground level, they slowly mixed upward 400
through Earth’s atmosphere. Because CFCs stay in the 1980 1985 1990
atmosphere for an average of 100 years, they eventually C CFC production Year
reached the stratosphere, where their concentrations
increased during the late 1900s. CFCs act as a green- FIGURE 18-9 Anthropogenic CFC increases Increasing
house gas, trapping outgoing back radiation from Earth’s CFC concentrations in the atmosphere in recent decades have
been the result of human production. (A, B) The total amount
surface. Their increase in the 1900s explains about 10% of ozone in a column of air over Antarctica in spring decreased
of the total greenhouse-gas warming. by half between the 1960s and 1980s because of rising CFC
Several decades ago, the biologist James Lovelock, levels. (C) The 1987 international Montreal agreement has
originator of the Gaia hypothesis, inferred that the ris- begun to reduce CFC production. (A: Adapted from T. E.
ing production of the CFCs might be causing dangerous Graedel and P. J. Crutzen, Atmosphere, Climate, and Change, Scientific
increases in their concentration in the stratosphere. In American Library, ©1997 by Lucent Technologies. B, C: Adapted
the 1970s, the atmospheric chemists Sherwood Roland from T. E. Graedel and P. J. Crutzen, Atmosphere, Climate, and
and Mario Molina confirmed that the CFCs were indeed Change, Scientific American Library, ©1997 by Lucent Technologies,
having a dangerous impact because of their interaction after J. C. Farman et al., “Large Losses of Total Ozone in Antarctica
with stratospheric ozone. Reveal Seasonal Interaction,” Nature 315 [1985]: 201–10.)