Page 143 - Electrical Installation in Hazardous Area
P. 143
Calculation of release rates and extents 109
The evaporation rate from a pool is given by the following equation:
G = A kg Ap M kg/s (Equation 4.18)
where G = vapourization rate kg/s
A = pool area m2
Ap = partial pressure of vapour over liquid
as a fraction of atmospheric pressure
kg = overall mass transfer coefficient
This equation can be converted into a volume vaporization rate by using
Equation 4.4 and results in the following volume vaporization rate:
Q = 8.2A kg ApT/102 m3/s (Equation 4.19)
where T = ambient temperature k
The partial pressure of vapour above a liquid is also known as its vapour
pressure and below boiling point is a fraction of atmospheric pressure,
which also indicates the ratio of vapour to air. Where this is not known an
estimation can often be made using the figures in Table 4.2.
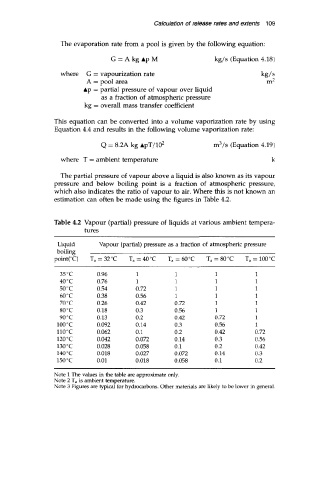
Table 4.2 Vapour (partial) pressure of liquids at various ambient tempera-
tures
Liquid Vapour (partial) pressure as a fraction of atmospheric pressure
boiling
point("C) T, = 32°C T, = 40°C T, = 60°C T, = 80°C T, = 100°C
35 "C 0.96 1 1 1 1
40 "C 0.76 1 1 1 1
50 "C 0.54 0.72 1 1 1
60 "C 0.38 0.56 1 1 1
70 "C 0.26 0.42 0.72 1 1
80 "C 0.18 0.3 0.56 1 1
90 "C 0.13 0.2 0.42 0.72 1
100°C 0.092 0.14 0.3 0.56 1
110 "C 0.062 0.1 0.2 0.42 0.72
120°C 0.042 0.072 0.14 0.3 0.56
130 "C 0.028 0.058 0.1 0.2 0.42
140 "C 0.018 0.027 0.072 0.14 0.3
150°C 0.01 0.018 0.058 0.1 0.2
Note 1 The values in the table are approximate only.
Note 2 T, is ambient temperature.
Note 3 Figures are typical for hydrocarbons. Other materials are likely to be lower in general.