Page 375 - Academic Press Encyclopedia of Physical Science and Technology 3rd Chemical Engineering
P. 375
P1: GLQ Final Pages
Encyclopedia of Physical Science and Technology EN009K-419 July 19, 2001 20:57
310 Membranes, Synthetic, Applications
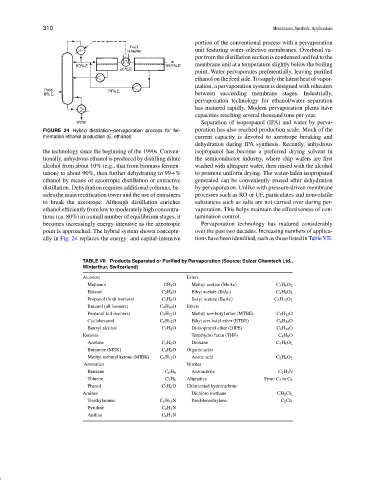
portion of the conventional process with a pervaporation
unit featuring water-selective membranes. Overhead va-
por from the distillation section is condensed and fed to the
membrane unit at a temperature slightly below the boiling
point. Water pervaporates preferentially, leaving purified
ethanol on the feed side. To supply the latent heat of vapor-
ization, a pervaporation system is designed with reheaters
between succeeding membrane stages. Industrially,
pervaporation technology for ethanol/water separation
has matured rapidly. Modern pervaporation plants have
capacities reaching several thousand tons per year.
Separation of isopropanol (IPA) and water by perva-
FIGURE 24 Hybrid distillation–pervaporation process for fer- poration has also reached production scale. Much of the
mentation ethanol production (E, ethanol). current capacity is devoted to azeotrope breaking and
dehydration during IPA synthesis. Recently, anhydrous
the technology since the beginning of the 1990s. Conven- isopropanol has become a preferred drying solvent in
tionally, anhydrous ethanol is produced by distilling dilute the semiconductor industry, where chip wafers are first
alcohol from about 10% (e.g., that from biomass fermen- washed with ultrapure water, then rinsed with the alcohol
tation) to about 90%, then further dehydrating to 99+% to promote uniform drying. The water-laden isopropanol
ethanol by means of azeotropic distillation or extractive generated can be conveniently reused after dehydration
distillation. Dehydration requires additional columns, be- by pervaporation. Unlike with pressure-driven membrane
sides the main rectification tower and the use of entrainers processes such as RO or UF, particulates and nonvolatile
to break the azeotrope. Although distillation enriches substances such as salts are not carried over during per-
ethanol efficiently from low to moderately high concentra- vaporation. This helps maintain the effectiveness of con-
tions (ca. 80%) in a small number of equilibrium stages, it tamination control.
becomes increasingly energy-intensive as the azeotropic Pervaporation technology has matured considerably
point is approached. The hybrid system shown conceptu- over the past two decades. Increasing numbers of applica-
ally in Fig. 24 replaces the energy- and capital-intensive tions have been identified, such as those listed in Table VII.
TABLE VII Products Separated or Purified by Pervaporation (Source: Sulzer Chemtech Ltd.,
Winterthur, Switzerland)
Alcohols Esters
Methanol CH 4 O Methyl acetate (MeAc) C 3 H 6 O 2
Ethanol C 2 H 6 O Ethyl acetate (EtAc) C 4 H 8 O 2
Propanol (both isomers) C 3 H 8 O Butyl acetate (BuAc) C 6 H 12 O 2
Butanol (all isomers) C 4 H 10 O Ethers
Pentanol (all isomers) C 5 H 12 O Methyl tert-butyl ether (MTBE) C 5 H 12 O
Cyclohexanol C 6 H 12 O Ethyl tert-butyl ether (ETBE) C 6 H 14 O
Benzyl alcohol C 7 H 8 O Di-isopropyl ether (DIPE) C 6 H 14 O
Ketones Tetrahydro furan (THF) C 4 H 8 O
Acetone C 3 H 6 O Dioxane C 4 H 8 O 2
Butanone (MEK) C 4 H 8 O Organic acids
Methyl isobutyl ketone (MIBK) C 6 H 12 O Acetic acid C 2 H 4 O 2
Aromatics Nitriles
Benzene C 6 H 6 Acetonitrile C 2 H 3 N
Toluene C 7 H 8 Aliphatics From C 3 to C 8
Phenol C 5 H 6 O Chlorinated hydrocarbons
Amines Dichloro methane CH 2 Cl 2
Triethylamine C 6 H 15 N Perchloroethylene C 2 Cl 4
Pyridine C 6 H 5 N
Aniline C 6 H 7 N