Page 409 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 409
P1: GLQ Final pages
Encyclopedia of Physical Science and Technology EN012C-568 July 26, 2001 15:32
Photoelectron Spectroscopy 59
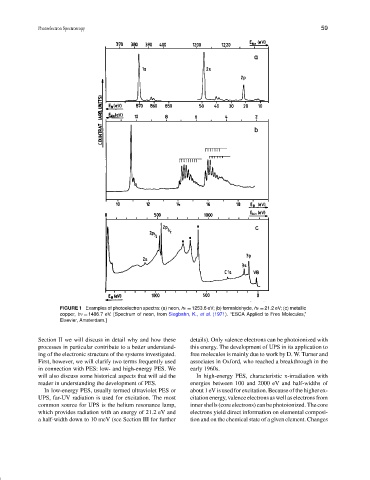
FIGURE 1 Examples of photoelectron spectra: (a) neon, hν = 1253.6 eV; (b) formaldehyde, hν = 21.2 eV; (c) metallic
copper, hν = 1486.7 eV. [Spectrum of neon, from Siegbahn, K., et al. (1971). “ESCA Applied to Free Molecules,”
Elsevier, Amsterdam.]
Section II we will discuss in detail why and how these details). Only valence electrons can be photoionized with
processes in particular contribute to a better understand- this energy. The development of UPS in its application to
ing of the electronic structure of the systems investigated. free molecules is mainly due to work by D. W. Turner and
First, however, we will clarify two terms frequently used associates in Oxford, who reached a breakthrough in the
in connection with PES: low- and high-energy PES. We early 1960s.
will also discuss some historical aspects that will aid the In high-energy PES, characteristic x-irradiation with
reader in understanding the development of PES. energies between 100 and 2000 eV and half-widths of
In low-energy PES, usually termed ultraviolet PES or about 1 eV is used for excitation. Because of the higher ex-
UPS, far-UV radiation is used for excitation. The most citation energy, valence electrons as well as electrons from
common source for UPS is the helium resonance lamp, inner shells (core electrons) can be photoionized. The core
which provides radiation with an energy of 21.2 eV and electrons yield direct information on elemental composi-
a half-width down to 10 meV (see Section III for further tion and on the chemical state of a given element. Changes