Page 412 - Academic Press Encyclopedia of Physical Science and Technology 3rd Analytical Chemistry
P. 412
P1: GLQ Final pages
Encyclopedia of Physical Science and Technology EN012C-568 July 26, 2001 15:32
62 Photoelectron Spectroscopy
with an average value of only a few angstroms. Even for a
kinetic energy of 10 or 1000 eV, the escape depth is only
˚
on the order of 20 A. PES probes only the few outermost
atomic layers of a solid, which can be a disadvantage if
one wants to study the bulk material. First, the composi-
tion of the surface is often different from the composition
of the bulk because of segregation effects or surface con-
tamination (Section III.A). Even if there is no difference
in composition, there is usually a strong contribution from
the outermost layer, especially for kinetic energies around
100 eV. The outermost layer is chemically always different
from the interior, since the atoms in this layer have fewer
neighbors. The surface sensitivity of PES is advantageous,
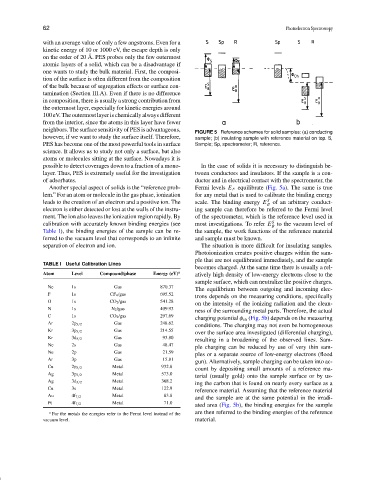
FIGURE 5 Reference schemes for solid samples: (a) conducting
however, if we want to study the surface itself. Therefore, sample; (b) insulating sample with reference material on top. S,
PES has become one of the most powerful tools in surface Sample; Sp, spectrometer; R, reference.
science. It allows us to study not only a surface, but also
atoms or molecules sitting at the surface. Nowadays it is
possible to detect coverages down to a fraction of a mono- In the case of solids it is necessary to distinguish be-
layer. Thus, PES is extremely useful for the investigation tween conductors and insulators. If the sample is a con-
of adsorbates. ductor and in electrical contact with the spectrometer, the
Another special aspect of solids is the “reference prob- Fermi levels E F equilibrate (Fig. 5a). The same is true
lem.” For an atom or molecule in the gas phase, ionization for any metal that is used to calibrate the binding energy
leads to the creation of an electron and a positive ion. The scale. The binding energy E S B of an arbitrary conduct-
electron is either detected or lost at the walls of the instru- ing sample can therefore be referred to the Fermi level
ment. The ion also leaves the ionization region rapidly. By of the spectrometer, which is the reference level used in
S
calibration with accurately known binding energies (see most investigations. To refer E to the vacuum level of
B
Table I), the binding energies of the sample can be re- the sample, the work functions of the reference material
ferred to the vacuum level that corresponds to an infinite and sample must be known.
separation of electron and ion. The situation is more difficult for insulating samples.
Photoionization creates positive charges within the sam-
ple that are not equilibrated immediately, and the sample
TABLE I Useful Calibration Lines
becomes charged. At the same time there is usually a rel-
Atom Level Compound/phase Energy (eV) a atively high density of low-energy electrons close to the
sample surface, which can neutralize the positive charges.
Ne 1s Gas 870.37
The equilibrium between outgoing and incoming elec-
F 1s CF 4 /gas 695.52
trons depends on the measuring conditions, specifically
O 1s CO 2 /gas 541.28
on the intensity of the ionizing radiation and the clean-
N 1s N 2 /gas 409.93
ness of the surrounding metal parts. Therefore, the actual
C 1s CO 2 /gas 297.69
charging potential φ ch (Fig. 5b) depends on the measuring
Ar 2p 3/2 Gas 248.62
conditions. The charging may not even be homogeneous
Kr 3p 3/2 Gas 214.55
over the surface area investigated (differential charging),
Kr 3d 5/2 Gas 93.80
resulting in a broadening of the observed lines. Sam-
Ne 2s Gas 48.47
ple charging can be reduced by use of very thin sam-
Ne 2p Gas 21.59
ples or a separate source of low-energy electrons (flood
Ar 3p Gas 15.81
gun). Alternatively, sample charging can be taken into ac-
Cu 2p 3/2 Metal 932.8
count by depositing small amounts of a reference ma-
Ag 3p 3/2 Metal 573.0
terial (usually gold) onto the sample surface or by us-
Ag 3d 5/2 Metal 368.2
ing the carbon that is found on nearly every surface as a
Cu 3s Metal 122.9
reference material. Assuming that the reference material
Au 4f 7/2 Metal 83.8
and the sample are at the same potential in the irradi-
Pt 4f 7/2 Metal 71.0
ated area (Fig. 5b), the binding energies for the sample
a are then referred to the binding energies of the reference
For the metals the energies refer to the Fermi level instead of the
vacuum level. material.