Page 115 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 115
P1: FMX/LSU P2: GPB/GRD P3: GLQ Final pages
Encyclopedia of Physical Science and Technology EN012c-593 July 26, 2001 15:56
Polymer Processing 621
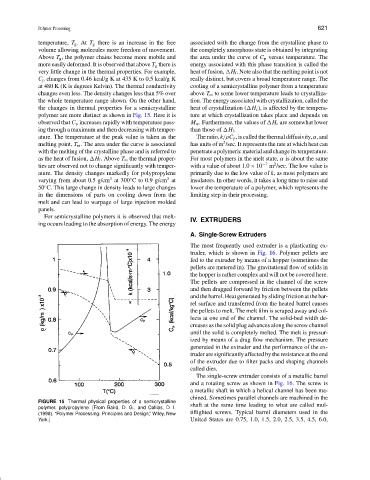
temperature, T g . At T g there is an increase in the free associated with the change from the crystalline phase to
volume allowing molecules more freedom of movement. the completely amorphous state is obtained by integrating
Above T g , the polymer chains become more mobile and the area under the curve of C p versus temperature. The
more easily deformed. It is observed that above T g there is energy associated with this phase transition is called the
very little change in the thermal properties. For example, heat of fusion, H f . Note also that the melting point is not
C p changes from 0.46 kcal/g K at 435 K to 0.5 kcal/g K really distinct, but covers a broad temperature range. The
at 480 K (K is degrees Kelvin). The thermal conductivity cooling of a semicrystalline polymer from a temperature
changes even less. The density changes less than 5% over above T m to some lower temperature leads to crystalliza-
the whole temperature range shown. On the other hand, tion. The energy associated with crystallization, called the
the changes in thermal properties for a semicrystalline heat of crystallization ( H c ), is affected by the tempera-
polymer are more distinct as shown in Fig. 15. Here it is ture at which crystallization takes place and depends on
observed that C p increases rapidly with temperature pass- M w . Furthermore, the values of H c are somewhat lower
ing through a maximum and then decreasing with temper- than those of H f .
ature. The temperature at the peak value is taken as the The ratio, k /ρC p , is called the thermal diffusivity, α, and
2
melting point, T m . The area under the curve is associated has units of m /sec. It represents the rate at which heat can
with the melting of the crystalline phase and is referred to penetrate a polymeric material and change its temperature.
as the heat of fusion, H f . Above T m the thermal proper- For most polymers in the melt state, α is about the same
2
ties are observed not to change significantly with temper- with a value of about 1.0 × 10 −7 m /sec. The low value is
ature. The density changes markedly for polypropylene primarily due to the low value of k, as most polymers are
3
3
varying from about 0.5 g/cm at 300 C to 0.9 g/cm at insulators. In other words, it takes a long time to raise and
◦
50 C. This large change in density leads to large changes lower the temperature of a polymer, which represents the
◦
in the dimensions of parts on cooling down from the limiting step in their processing.
melt and can lead to warpage of large injection molded
panels.
For semicrystalline polymers it is observed that melt-
IV. EXTRUDERS
ing occurs leading to the absorption of energy. The energy
A. Single-Screw Extruders
The most frequently used extruder is a plasticating ex-
truder, which is shown in Fig. 16. Polymer pellets are
fed to the extruder by means of a hopper (sometimes the
pellets are metered in). The gravitational flow of solids in
the hopper is rather complex and will not be covered here.
The pellets are compressed in the channel of the screw
and then dragged forward by friction between the pellets
and the barrel. Heat generated by sliding friction at the bar-
rel surface and transferred from the heated barrel causes
the pellets to melt. The melt film is scraped away and col-
lects at one end of the channel. The solid-bed width de-
creases as the solid plug advances along the screw channel
until the solid is completely melted. The melt is pressur-
ized by means of a drag flow mechanism. The pressure
generated in the extruder and the performance of the ex-
truder are significantly affected by the resistance at the end
of the extruder due to filter packs and shaping channels
called dies.
The single-screw extruder consists of a metallic barrel
and a rotating screw as shown in Fig. 16. The screw is
a metallic shaft in which a helical channel has been ma-
chined. Sometimes parallel channels are machined in the
FIGURE 15 Thermal physical properties of a semicrystalline
polymer, polypropylene. [From Baird, D. G., and Collias, D. I. shaft at the same time leading to what are called mul-
(1998). “Polymer Processing: Principles and Design,” Wiley, New tiflighted screws. Typical barrel diameters used in the
York.] United States are 0.75, 1.0, 1.5, 2.0, 2.5, 3.5, 4.5, 6.0,