Page 43 - Engineered Interfaces in Fiber Reinforced Composites
P. 43
26 Engineered interfaces in jiber reinforced composites
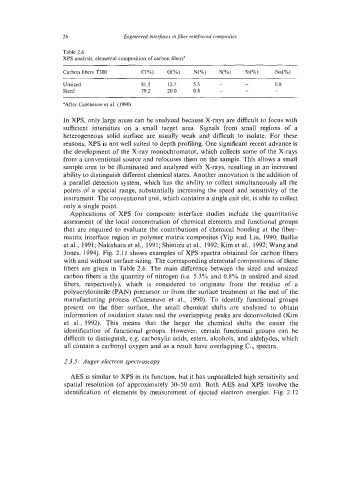
Table 2.6
XPS analysis, elemental composition of carbon fibers"
Carbon fibers T300 C(%) O(%) N(%) S(%) Si(%) Na(%)
Unsized 81.5 12.7 5.3 - - 0.8
Sized 79.2 20.0 0.8 - - -
"After Cazeneuve et al. (1990)
In XPS, only large areas can be analyzed because X-rays are difficult to focus with
sufficient intensities on a small target area. Signals from small regions of a
heterogeneous solid surface are usually weak and difficult to isolate. For these
reasons, XPS is not well suited to depth profiling. One significant recent advance is
the development of the X-ray monochromator, which collects some of the X-rays
from a conventional source and refocuses them on the sample. This allows a small
sample area to be illuminated and analyzed with X-rays, resulting in an increased
ability to distinguish different chemical states. Another innovation is the addition of
a parallel detection system, which has the abiIity to collect simultaneously all the
points of a special range, substantially increasing the speed and sensitivity of the
instrument. The conventional unit, which contains a single exit slit, is able to collect
only a single point.
Applications of XPS for composite interface studies include the quantitative
assessment of the local concentration of chemical elements and functional groups
that are required to evaluate the contributions of chemical bonding at the fiber-
matrix interface region in polymer matrix composites (Yip and Liu, 1990; Baillie
et al., 1991; Nakahara et al., 1991; Shimizu et al., 1992; Kim et al., 1992; Wang and
Jones, 1994). Fig. 2.1 1 shows examples of XPS spectra obtained for carbon fibers
with and without surface sizing. The corresponding elemental compositions of these
fibers are given in Table 2.6. The main difference between the sized and unsized
carbon fibers is the quantity of nitrogen (Le. 5.3% and 0.8% in unsized and sized
fibers, respectively), which is considered to originate from the residue of a
polyacrylonitrile (PAN) precursor or from the surface treatment at the end of the
manufacturing process (Cazeneuve et al., 1990). To identify functional groups
present on the fiber surface, the small chemical shifts are analyzed to obtain
information of oxidation states and the overlapping peaks are deconvoluted (Kim
et al., 1992). This means that the larger the chemical shifts the easier the
identification of functional groups. However, certain functional groups can be
difficult to distinguish, e.g. carboxylic acids, esters, alcohols, and aldehydes, which
all contain a carbonyl oxygen and as a result have overlapping C1, spectra.
2.3.5. Auger electron spectroscopy
AES is similar to XPS in its function, but it has unparalleled high sensitivity and
spatial resolution (of approximately 30-50 nm). Both AES and XPS involve the
identification of elements by measurement of ejected electron energies. Fig. 2.12