Page 45 - Engineered Interfaces in Fiber Reinforced Composites
P. 45
28 Engineered interfaces in fiber reinforced composites
SIMS and
XPS ISS Ion
Auger Electron
Elec ‘&on Excitation
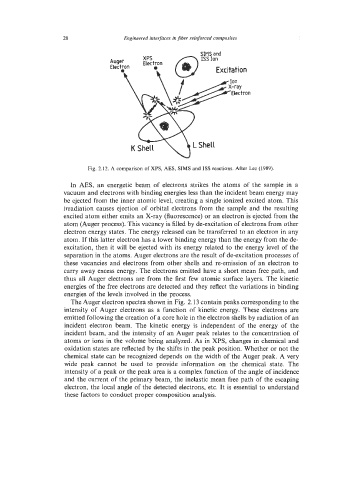
Fig. 2.12. A comparison of XPS, AES, SIMS and ISS reactions. After Lee (1989).
In AES, an energetic beam of electrons strikes the atoms of the sample in a
vacuum and electrons with binding energies less than the incident beam energy may
be ejected from the inner atomic level, creating a single ionized excited atom. This
irradiation causes ejection of orbital electrons from the sample and the resulting
excited atom either emits an X-ray (fluorescence) or an electron is ejected from the
atom (Auger process). This vacancy is filled by de-excitation of electrons from other
electron energy states. The energy released can be transferred to an electron in any
atom. If this latter electron has a lower binding energy than the energy from the de-
excitation, then it will be ejected with its energy related to the energy level of the
separation in the atoms. Auger electrons are the result of de-excitation processes of
these vacancies and electrons from other shells and re-emission of an electron to
carry away excess energy. The electrons emitted have a short mean free path, and
thus all Auger electrons are from the first few atomic surface layers. The kinetic
energies of the free electrons are detected and they reflect the variations in binding
energies of the levels involved in the process.
The Auger electron spectra shown in Fig. 2.13 contain peaks corresponding to the
intensity of Auger electrons as a function of kinetic energy. These electrons are
emitted following the creation of a core hole in the electron shells by radiation of an
incident electron beam. The kinetic energy is independent of the energy of the
incident beam, and the intensity of an Auger peak relates to the concentration of
atoms or ions in the volume being analyzed. As in XPS, changes in chemical and
oxidation states are reflected by the shifts in the peak position. Whether or not the
chemical state can be recognized depends on the width of the Auger peak. A very
wide peak cannot be used to provide information on the chemical state. The
intensity of a peak or the peak area is a complex function of the angle of incidence
and the current of the primary beam, the inelastic mean free path of the escaping
electron, the local angle of the detected electrons, etc. It is essential to understand
these factors to conduct proper composition analysis.