Page 144 - Entrophy Analysis in Thermal Engineering Systems
P. 144
138 Entropy Analysis in Thermal Engineering Systems
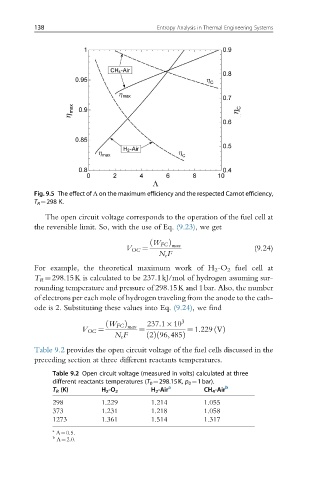
Fig. 9.5 The effect of Λ on the maximum efficiency and the respected Carnot efficiency,
T R ¼298 K.
The open circuit voltage corresponds to the operation of the fuel cell at
the reversible limit. So, with the use of Eq. (9.23), we get
ð W FC Þ
V OC ¼ max (9.24)
N e F
For example, the theoretical maximum work of H 2 -O 2 fuel cell at
T R ¼298.15K is calculated to be 237.1kJ/mol of hydrogen assuming sur-
rounding temperature and pressure of 298.15K and 1bar. Also, the number
of electrons per each mole of hydrogen traveling from the anode to the cath-
ode is 2. Substituting these values into Eq. (9.24), we find
ð W FC Þ 237:1 10 3
V OC ¼ max ¼ ¼ 1:229 VðÞ
2 ðÞ 96;485Þ
ð
N e F
Table 9.2 provides the open circuit voltage of the fuel cells discussed in the
preceding section at three different reactants temperatures.
Table 9.2 Open circuit voltage (measured in volts) calculated at three
different reactants temperatures (T 0 ¼298.15K, p 0 ¼1bar).
T R (K) H 2 -O 2 H 2 -Air a CH 4 -Air b
298 1.229 1.214 1.055
373 1.231 1.218 1.058
1273 1.361 1.514 1.317
a
Λ¼0.5.
b
Λ¼2.0.