Page 143 - Entrophy Analysis in Thermal Engineering Systems
P. 143
Entropy and fuel cells 137
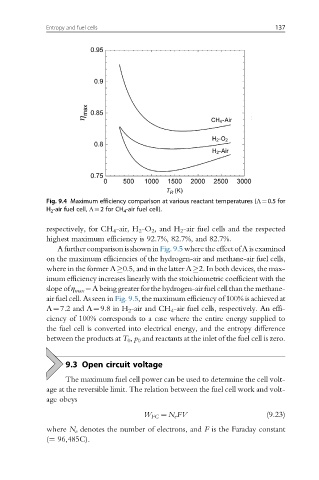
Fig. 9.4 Maximum efficiency comparison at various reactant temperatures (Λ¼0.5 for
H 2 -air fuel cell, Λ¼2 for CH 4 -air fuel cell).
respectively, for CH 4 -air, H 2 -O 2 , and H 2 -air fuel cells and the respected
highest maximum efficiency is 92.7%, 82.7%, and 82.7%.
A further comparison is showninFig.9.5 wherethe effectofΛ is examined
on the maximum efficiencies of the hydrogen-air and methane-air fuel cells,
where in the former Λ 0.5, and in the latter Λ 2. In both devices, the max-
imum efficiency increases linearly with the stoichiometric coefficient with the
slopeofη max Λ beinggreater forthehydrogen-air fuel cellthan the methane-
air fuel cell. As seen in Fig. 9.5, the maximum efficiency of 100% is achieved at
Λ¼7.2 and Λ¼9.8inH 2 -air and CH 4 -air fuel cells, respectively. An effi-
ciency of 100% corresponds to a case where the entire energy supplied to
the fuel cell is converted into electrical energy, and the entropy difference
between the products at T 0 , p 0 and reactants at the inlet of the fuel cell is zero.
9.3 Open circuit voltage
The maximum fuel cell power can be used to determine the cell volt-
age at the reversible limit. The relation between the fuel cell work and volt-
age obeys
(9.23)
W FC ¼ N e FV
where N e denotes the number of electrons, and F is the Faraday constant
(¼ 96,485C).