Page 82 - Fundamentals of Gas Shale Reservoirs
P. 82
62 GEOCHEMICAL ASSESSMENT OF UNCONVENTIONAL SHALE GAS RESOURCE SYSTEMS
–80.0
Barnett Shale (C vs C ) 2
1
–75.0 Fayetteville Shale (C vs C )
1
2
3
–70.0 Biogenic gas 90% 80% Barnett Shale (C vs C ) 2 2
Fayetteville Shale (C vs C )
3
13 C methane, propane (per million) –55.0 Rollover 50%
–65.0
70%
–60.0
60%
40%
–50.0
30%
–45.0
20%
–40.0
10%
–35.0
13
13
1
–30.0
–25.0 C vs C 2
13
13
–20.0 0.80% 1.50% 2.30% C vs C 2
3
Roe=0.70% 1.10% 1.80% 3.20%
–15.0
–50.0 –45.0 –40.0 –35.0 –30.0 –25.0 –20.0 –15.0
13
C ethane (per million)
13
13
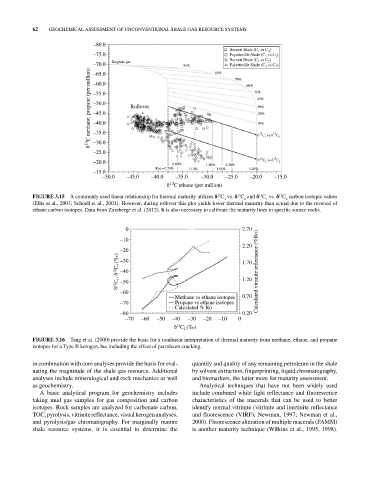
FIGURE 3.15 A commonly used linear relationship for thermal maturity utilizes δ C vs. δ C and δ C vs. δ C carbon isotopic values
13
13
1
3
2
2
(Ellis et al., 2003; Schoell et al., 2001). However, during rollover this plot yields lower thermal maturity than actual due to the reversal of
ethane carbon isotopes. Data from Zumberge et al. (2012). It is also necessary to calibrate the maturity lines to specific source rocks.
0 2.70
–10
2.20
–20
13 C 1 , 13 C 3 (‰) –40 1.70 Calculated vitrinite re ectance (%Ro)
–30
1.20
–50
–60
Methane vs ethane isotopes 0.70
–70 Propane vs ethane isotopes
Calculated % Ro
–80 0.20
–70 –60 –50 –40 –30 –20 –10 0
C (‰)
13
2
FIGURE 3.16 Tang et al. (2000) provide the basis for a nonlinear interpretation of thermal maturity from methane, ethane, and propane
isotopes for a Type II kerogen, but including the effect of petroleum cracking.
in combination with core analyses provide the basis for eval quantity and quality of any remaining petroleum in the shale
uating the magnitude of the shale gas resource. Additional by solvent extraction, fingerprinting, liquid chromatography,
analyses include mineralogical and rock mechanics as well and biomarkers, the latter more for maturity assessment.
as geochemistry. Analytical techniques that have not been widely used
A basic analytical program for geochemistry includes include combined white light reflectance and fluorescence
taking mud gas samples for gas composition and carbon characteristics of the macerals that can be used to better
isotopes. Rock samples are analyzed for carbonate carbon, identify normal vitrinite (vitrinite and inertinite reflectance
TOC, pyrolysis, vitrinite reflectance, visual kerogen analyses, and fluorescence (VIRF); Newman, 1997; Newman et al.,
and pyrolysis/gas chromatography. For marginally mature 2000). Fluorescence alteration of multiple macerals (FAMM)
shale resource systems, it is essential to determine the is another maturity technique (Wilkins et al., 1995, 1998).